Tumor-derived exosomes: immune properties and clinical application in lung cancer
Abstract
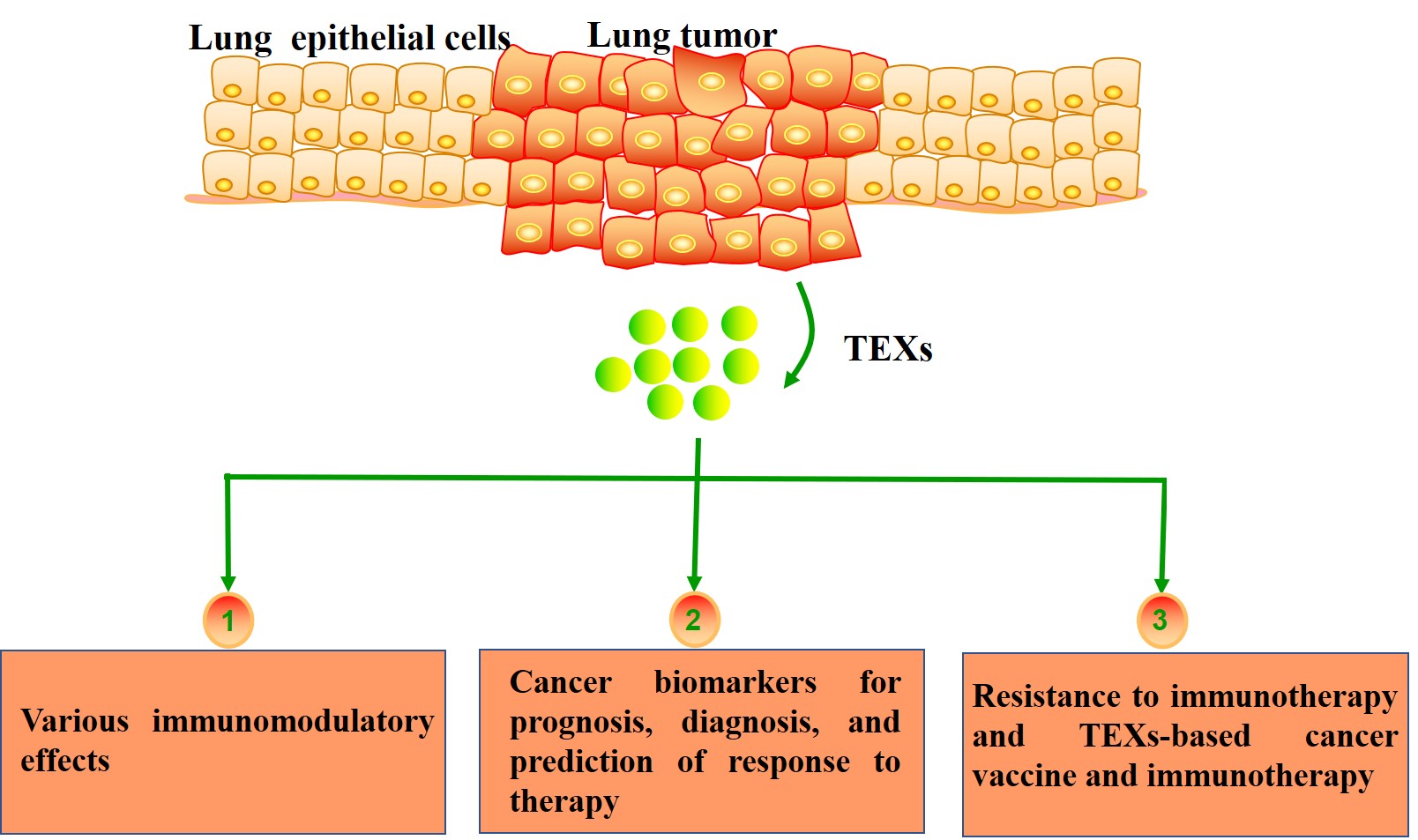
Lung cancer is the leading cause of cancer-related death worldwide. Despite advances in diagnosis and treatment of lung cancer, the overall survival remains poor. Evidence indicates that lung cancer development is a complex and dynamic process that involves interactions between tumor cells and their microenvironments, including immune cells. Exosomes are small extracellular vesicles secreted by most cell types; they contain functional molecules that allow intercellular communication. Tumor-derived exosomes (TEXs) carry both immunosuppressive and immunostimulatory mediators and may be involved in various immunomodulatory effects. TEXs, which partially mimic profiles of the parent cells, are a potential source of cancer biomarkers for prognosis, diagnosis, and prediction of response to therapy. In addition, TEXs may interfere with immunotherapies, but they also could be used as adjuvants and antigenic components in vaccines against lung cancer. In the context of lung cancer, identifying TEXs and understanding their contribution to tumorigenesis and the response to immunotherapies represents a challenging research area.
Keywords
INTRODUCTION
Lung cancer is one of the most common malignant tumors with the highest morbidity and mortality worldwide[1]. Recently, immunotherapies have shown more effectiveness than traditional chemotherapy, and they have dramatically changed the treatment paradigm for lung cancer[2-4]. However, only a small proportion of patients can benefit from the immunotherapies, with primary and secondary resistance complicating treatment. One potential explanation for this phenomenon is the complexity and diversity of the tumor microenvironment (TME). Interactions of lung cancer cells with the surrounding TME are critical to cancer progression and response to immunotherapy. In recent years, the communication mediated by exosomes has extensively gained attention.
Exosomes are small bilayer membrane vesicles with a size of 30-100 nm in diameter that are secreted by various cell types such as tumor cells, immune cells, and fibroblasts[5,6]. Exosomes derived from tumor cells are referred to as tumor-derived exosomes (TEXs)[7]. TEXs have been shown to contain a variety of biomolecules including nucleic acids, proteins, enzymes, and lipids, which are involved in cancer progression, intercellular communication, and immunological function[8]. Studies have increasingly indicated that the number and composition of TEXs can in part reflect their cells of origin and biological state, which may serve as potential biomarkers in diagnosis and prognosis of cancer[9-11]. TEXs may affect cancer immunotherapy either by sequestration of therapeutic antibodies or supplying self-antigen carriers to improve cancer vaccine efficacy. Their biological roles in cancer progression as well as cancer immunotherapy and biomarkers have indicated that TEXs are critical components of the TME.
In this review, we first describe how TEXs are formed and released to the extracellular matrix, and discuss the composition of TEXs. Then, we outline the immunomodulatory function of TEXs in the lung cancer microenvironment. Moreover, we focus on the utility of TEXs as diagnostic and prognostic biomarkers in lung cancer. Finally, the recent findings on TEXs in immunological changes during immunotherapy are discussed.
THE FORMATION, RELEASE, AND COMPOSITION OF TEXS
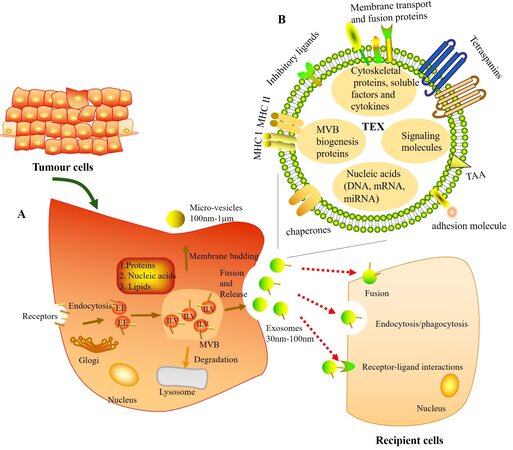
TEXs, released by tumor cells, are present ubiquitously in tumor tissues and body fluids[12]. Exosome biogenesis initiates from the production of early endosomes via the internalization of membrane microdomains. The limiting membrane of early endosomes bud inwardly to form intraluminal vesicles, then becoming the multivesicular bodies. Finally, exosomes are released when multivesicular bodies fuse with the plasma membrane[13]. This formation of exosomes is a tightly regulated process; it involves two pathways through an Endosomal Sorting Complex Required for Transport (ESCRT)-dependent machinery or an ESCRT-independent machinery[14]. Once exosomes released, they are able to transfer information to their recipient cells through three main ways: endocytosis/phagocytosis, direct fusion with cellular membrane, and receptor-ligand interactions[13] [Figure 1A].
Figure 1. Molecular composition, biogenesis, release, and uptake of tumor-derived exosomes (TEXs). (A) TEXs originate from intraluminal vesicles (ILVs) in the multivesicular bodies (MVBs) (also known as late endosomes). Firstly, early endosomes (EEs) are formed when the membrane microdomains are endocytosed via inward budding of the plasma membrane. Then EEs mature into MVBs, which follow either fusion with the plasma membrane to form exosomes or degradation by lysosome. During this process, the proteins, nucleic acids, and lipids are packed into exosomes. Finally, exosomes can interact with recipient cells through three main ways: endocytosis/phagocytosis, direct fusion with cellular membrane, and receptor-ligand interactions. (B) Schematic diagram of components of TEXs.
TEXs consist of a lipid-protein bilayer membrane, including membrane transport and fusion proteins (e.g., annexins, Rab proteins, and flotillin), MHC (class I and II molecules), adhesion molecules (e.g., ICAM, EPCAM, CD44, and integrins), inhibitory ligands [e.g., FasL, TRAIL, PD-L1, and transforming growth factor (TGF)-β/LAP], tetraspanins (e.g., CD9, CD63, CD81, and CD82), tumor associated antigens, chaperones [e.g., heat-shock protein (HSP) 70 and HSP90], lipids, and glycolipids[12,13,15-17]. In their lumen, TEXs carry a variety of multivesicular bodies biogenesis proteins [e.g., Alix and tumor susceptibility gene 101 (TSG101)], cytoskeletal proteins (e.g., actin, tubulin, and vimentin), histones, oncoproteins, soluble factors, enzymes, cytokines, signaling molecules, and nucleic acids (including DNA, mRNA, and miRNA)[13,15] [Figure 1B]. The TEXs molecular and genetic content mimics that of parent cells, and is in part considered as surrogates of the parent tumor cells[18]. Moreover, TEXs can transfer messages from the parent tumor to recipient cells, including immune cells, within the TME[19].
EFFECT OF TEXS FOR IMMUNE REGULATION IN LUNG CANCER
Recently, TEXs have been proposed to act as crucial mediators between cellular communication by transferring both immunosuppressive and immunostimulatory signals to immune cells in a lung cancer microenvironment[20,21] [Table 1, Figure 2].
Figure 2. Tumor-derived exosomes (TEXs) carry and deliver both immunosuppressive and immunostimulatory signals to immune cells in the lung tumor microenvironment. (A) Immune suppression. TEXs contribute to establish an immunosuppressive TME by inducing apoptosis and inhibiting the activity of effector T cells, skewing M2 polarization of macrophages, expanding myeloid-derived suppressor cells (MDSCs), suppressing DCs differentiation, and impairing the function of NK cells. (B) Immune stimulation. TEXs can also stimulate immune cells to support antitumor activities, including enhancing the activity of macrophages and NK cells, suppressing M2 macrophage polarization, and increase T cells activity directly or indirectly.
Overview of exosomal cargo, source of exosomes, and their biological effects
| Exosomal cargo | Donor | Biological effect | Ref. |
| PD-L1 | H1299, H358, and H1264 | Inactivate T cells | [23] |
| EGFR | NSCLC biopsies | Induce tolerogenic DCs | [28] |
| miR-214 | Lewis lung carcinoma cells | Downregulate the PTEN-mediated signaling | [29] |
| miR-433 | Plasma of NSCLC patients | Inactivate the WNT/β‑catenin signaling | [31] |
| miR-21/29a | A-549 and SK-MES | Activate TLR7 and TLR8 | [32] |
| miR-21a | Lewis lung carcinoma cells | Promote MDSCs expansion | [51] |
| miR-103a | CL1-5 lung cancer cells | Activate of PI3K/Akt and STAT3 signaling pathways | [52] |
| circFARSA | A549 and PC9 cells | Polarize macrophages to the M2 phenotype | [54] |
| miR-770 | A549 cells | Suppress M2 macrophage polarization | [62] |
Immunosuppressive effect of TEXs
TEXs modulate the activity of T cells by promoting apoptosis and inhibiting proliferation of CD8+ T cells[22]. Recent studies have indicated that TEXs contain PD-L1, which inhibits T cells activity and promotes tumor progression. Exosomes from lung cancer, melanoma, and breast cancer carry PD-L1 on their surface, which interacts with PD-1 via the extracellular domain, and thereby inactivate T cells[23]. Poggio et al.[24] discovered that the majority of PD-L1 could be presented on the surface of TEXs, and genetic blockade of exosomal PD-L1 could activate an anti-tumor immune response leading to extend survival in a subset of cancer patients. Moreover, it is suggested that TEXs with FasL expression could induce CD8+ T cell apoptosis[25,26]. Czystowska et al.[27] uncovered the mechanism that the PI3K/Akt pathway was a central target for TEXs in regulating CD8+ T cell apoptosis. Huang et al.[28] indicated that about 80% of exosomes isolated from non-small cell lung cancer (NSCLC) biopsies contained EGFR. These exosomes can be captured by dendritic cells (DCs). Then tolerogenic DCs were generated and induced tumor antigen specific regulatory T cells (Tregs), which could inhibit the function of tumor specific CD8+ T cells. Yin et al.[29] found that miR-214 was delivered into recipient CD4+ T cells via TEXs, so as to downregulate the PTEN-mediated signaling, thereby promoting Treg expansion and tumor growth. Interestingly, co-incubation of Treg with TEXs may enhance Treg number as well as its suppressive function with the increased production of inhibitory cytokines, TGF-β1 and interleukin (IL)-10[30]. Additionally, Liu et al.[31] showed that exosome-derived miR-433 inactivated the WNT/β-catenin signaling pathway via targeting transmembrane p24 trafficking protein 5, thus increasing infiltration of CD4+ and CD8+ cells in NSCLC.
Exosome-derived miR-21/29a derived from A-549 and SK-MES cells promoted lung cancer growth and metastasis through activating Toll like receptors TLR7 and TLR8 on immune cells including NK cells[32]. NK cells express a variety of receptors that are either stimulatory or inhibitory[33]. The downregulation of those stimulatory receptors, particularly NKG2D, may play an important role in decreasing activity of NK cells in lung cancer patients[34]. TEXs originating from hypoxic tumor cells deliver TGF-β1 to NK cells, and thereby reduce NKG2D expression resulting in lower activity of NK cells[35]. TEXs can also attenuate NK cell activity via multiple mechanisms including shedding the NKG2D ligand on tumor cells, suppressing Janus kinase (Jak) 3 activation, inhibiting perforin or cyclin D3 production and down-regulation of IL-2-mediated pathways[36-41]. Moreover, TEX-carried MICA and MICB ligands can downregulate the stimulatory receptors, especially NKG2D on NK cells[42].
TEXs suppressed the functioning of the immune system by affecting the monocyte differentiation and maturation[43,44]. TEXs were capable of blocking DCs migration to lymph nodes through inhibiting most
Taken together, these data suggested that TEXs can modulate the immune response by transferring immunosuppressive signals to immune cells, which in turn contribute to tumor progression[7,34,55,56].
Immunostimulatory effect of TEXs
TEXs have been reported to be involved in the suppression of the immune system in previous studies. However, since TEXs also carry stimulatory molecules that contribute to activating immune responses, recent studies also focused on anti-tumor immunity of exosomes.
TEXs can act as presenters participating in direct and indirect antigen presentation[57]. As direct presenters, TEXs present antigen to T cells via an MHC-peptide complex on their surface. On the other hand, TEXs can also indirectly transfer tumor antigen to antigen presenting cells, like DCs, and then activate the cytotoxic activity of CD8+ T cells and CD4+ T helper cells, so as to inhibit tumor growth[58]. Additionally, the enrichment of HSPs on TEXs such as Hsp70, can stimulate the activity of NK cells[59] and macrophages[60], and induce MHC class I-restricted cytotoxic T cells activation[61]. Tumor cell-derived exosomal miR-770 could suppress M2 macrophage polarization via targeting MAP3K1, which in turn decreased NSCLC tumor growth[62]. Tetraspanins on the exosome surface mainly mediate cell adhesion and participate in maintaining the optimal conformation of immune proteins like MHC class II, thus playing an important role in antitumor immunity through exosomal targeting to DCs[61,63-65].
Therefore, the effect of exosomes’ immune stimulation depends mainly on their antigen presentation, while the effect of inhibiting immunity mainly depends on exosome-carried biological content consisting of ligands, miRNAs, and proteins, which may inhibit the cytotoxic activity of the NK and CD8+ T cells or increase suppressive immune cells such as MDSCs, Treg cells, and M2 macrophages. Understanding the effect of TEXs in immune regulation will allow for better understanding of the clinical application of TEXs in cancer diagnosis and treatment.
TEXS SERVE AS DIAGNOSTIC AND PROGNOSTIC BIOMARKERS IN LUNG CANCER
TEXs and their content in biofluids, which represent the content of parent cells, may serve as newly developed non-invasive biomarkers for diagnosis, prognosis, and monitoring the efficacy of treatment in lung cancer[9-11].
Jakobsen et al.[66] examined the potential of exosomal proteins as diagnostic markers in advanced NSCLC. The EV (extracellular vesicle) array showed that the expression levels of CD9, CD63, and CD81 were significantly high in cancerous patients. Likewise, according to the EV array, NYESO-1, EGFR, and PLAP showed a strong correlation with a poor survival in NSCLC[11]. In addition, SRGN, TPM3, THBS1, and HUWE1 may serve as biomarkers to distinguish lung adenocarcinoma subjects from controls[67]. Combination of carcinoembryonic antigen, exosomal alpha-2-HS-glycoprotein and extracellular matrix protein 1 (ECM1) could improve the diagnostic accuracy of NSCLC[68]. Gao et al.[69] indicated that plasma exosomal total protein, Tim-3 and Galectin-9 were significantly increased in NSCLC, and were positively associated with larger tumor size, advanced TNM stage, and distant metastases. The higher level of leucinerich a-2-glycoprotein (LRG1) was detected in urinary exosomes and may be a non-invasive diagnostic biomarker of NSCLC in urine[70].
Several studies have shown that exosomal miRNAs may serve as potential biomarkers for the early diagnosis of lung cancer. Rabinowits et al.[71] suggested that exosomal miRNAs in NSCLC patients very closely resemble those in NSCLC tissue, indicating that such a liquid biopsy may obviate the need to obtain tumor tissues. Tumor-derived exosomal miRNAs, adenocarcinoma-specific miR-181-5p, miR-361-5p,
Recently, exosomal RNAs have been reported to predict the prognosis of a variety of cancers including lung cancer. Dejima et al.[78] showed that exosomal miR-21 and miR-4257 levels of the NSCLC patients were significantly upregulated during recurrence and can be used as recurrence-specific biomarkers. Additionally, another study reported that low exosomal let-7a-5p levels were significantly associated with a worse cancer-related survival rate in lung adenocarcinoma patients[79]. Luo et al.[80] also demonstrated that serum exosomal miR-382 was considered as an independent prognostic biomarker for NSCLC.
Acquired resistance to general therapies, including chemotherapy, radiotherapy, immunotherapy, and targeted therapy, is a major challenge in the treatment of lung cancer. Nowadays, the use of exosomes as biomarkers for predicting therapeutic responses has gathered much attention. Exosomal hsa_circ_0014235 isolated from plasma promoted cisplatin chemoresistance and may serve as a promising biomarker for NSCLC treatment[83]. Exosomal miR-4443 might also promote cisplatin resistance of NSCLC by regulating FSP1-mediated ferroptosis[84]. In addition, Li et al.[85] showed that plasma exosomal miR-92b-3p was significantly increased in chemoresistant small cell lung cancer patients and might serve as a potential dynamic biomarker for monitoring the drug resistance. Exosomal miR-29a-3p and miR-150-5p were identified as circulating biomarkers during thoracic radiation therapy for NSCLC and were correlated with delivered radiation therapy dose[86]. At present, precision medicine based on immunotherapy and targeted therapy has given new hope for lung cancer patients. The ensuing problems of drug resistance have gained much interest from the research community. For example, exosomal miR-323-3p, miR-1468-3p,
THE ROLE OF TEXS IN IMMUNOTHERAPY OF LUNG CANCER
Effect of TEXs for resistance to immunotherapy
TEXs may suppress proliferation and differentiation of immune cells and have the ability to influence their biological function. TEXs carrying a variety of tumor associated antigens or immunoinhibitory mediators not only suppress antitumor functions of immune effector cells, but also appear to impede effective response to immunotherapy in cancer[8]. Tumor associated antigens on TEXs could efficiently bind antibodies produced against cancer cells and block the access of therapeutic antibodies to the cancer cells, leading to a decrease in effectiveness of cancer therapy[91]. Additionally, TEXs are able to inhibit antibody dependent cell-mediated cytotoxicity, which serves as a critical mechanism of therapeutic antitumor activity of anticancer antibodies[91]. During therapy, immune escape in NSCLC occurs through a multistep process that facilitates tumor growth and progression. Acquired tumor resistance to immunotherapy could be directly reflected in the production of TEXs[92]. Most importantly, Kim et al.[93] showed that lung cancer cells increased their production of immunosuppressive exosomes during acquired resistance to anti-PDL1 immunotherapy.
TEXs-based cancer vaccine and immunotherapy
TEXs are nanoscale membrane-derived vesicles that are thought to be important mediators of intercellular communication. Moreover, TEXs with distinct characteristics such as stability, permeability, biocompatibility, low immunogenicity, and low toxicity can efficiently deliver tumor antigens to DCs, thus they can be used as self-antigen carriers to stimulate immune response[94-96]. Increasing evidences have demonstrated that the activation and maturation of DCs by TEXs could enhance anti-tumor effects and may be applied for lung cancer immunotherapy. For example, TEXs from CD40L-gene modified 3LL lung tumor cells have the potent ability to activate DCs, resulting in significantly increased tumor antigen-specific CD4+ T cell proliferation and CD8+ T cell responses, revealing a powerful antitumor effect[97]. In addition, the exosomes derived from Rab27-overexpressing NSCLC cells also stimulated the proliferation and maturation of DCs effectively, promoted CD4+ T cell proliferation and elicited potent antitumor immune responses[98]. Multiple studies have already proved that TEXs which were used as tumor antigens source for DC vaccines, have greater efficacy and safety than conventional tumor cell lysates[99-103].
CONCLUSION
TEXs are important mediators of intercellular communication and have been proven to play a key role in the TME. The biogenesis and secretion of TEXs have been widely reported. They carry a variety of cargoes and are involved in both immunosuppressive and immunostimulatory signaling pathways by delivering molecular signals to immune cells. The small size of TEXs and their contents render them highly interesting for biomedical applications, such as biomarker molecules and anticancer vaccines. Based on the data herein, we suggest that TEXs could be manipulated to provide clinical benefits and improve the clinical management of lung cancer.
DECLARATIONS
Authors’ contributionsManuscript writing: Wu J
Manuscript revision: Li S
Study design: Zhang P
All authors read and approved the final manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54.
2. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 2020;38:1505-17.
3. Mok TSK, Wu Y, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30.
4. Cao C, Guo A, Chen C, Zielinski R, Bott M. Neoadjuvant immunotherapy for patients with non-small cell lung cancer-current evidence. Ann Transl Med 2020;8:1476.
5. Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer 2020;19:160.
6. Zhu L, Sun HT, Wang S, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol 2020;13:152.
7. Olejarz W, Dominiak A, Żołnierzak A, Kubiak-Tomaszewska G, Lorenc T. Tumor-derived exosomes in immunosuppression and immunotherapy. J Immunol Res 2020;2020:6272498.
8. Whiteside TL. . Tumor-derived exosomes and their role in cancer progression. Elsevier; 2016. p. 103-41.
10. Sharma S, Salomon C. . Techniques associated with exosome isolation for biomarker development: liquid biopsies for ovarian cancer detection. In: Thurin M, Cesano A, Marincola FM, editors. Biomarkers for immunotherapy of cancer. New York: Springer; 2020. p. 181-99.
11. Sandfeld-Paulsen B, Aggerholm-Pedersen N, Bæk R, et al. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol Oncol 2016;10:1595-602.
12. Tian X, Shen H, Li Z, Wang T, Wang S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J Hematol Oncol 2019;12:84.
13. Xie F, Zhou X, Fang M, et al. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci (Weinh) 2019;6:1901779.
14. Salimi L, Akbari A, Jabbari N, et al. Synergies in exosomes and autophagy pathways for cellular homeostasis and metastasis of tumor cells. Cell Biosci 2020;10:64.
15. Taghikhani A, Farzaneh F, Sharifzad F, Mardpour S, Ebrahimi M, Hassan ZM. Engineered tumor-derived extracellular vesicles: potentials in cancer immunotherapy. Front Immunol 2020;11:221.
17. Czystowska-Kuzmicz M, Whiteside TL. The potential role of tumor-derived exosomes in diagnosis, prognosis, and response to therapy in cancer. Expert Opin Biol Ther 2021;21:241-58.
18. Whiteside TL. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol 2017;13:2583-92.
19. Whiteside TL. The emerging role of plasma exosomes in diagnosis, prognosis and therapies of patients with cancer. Contemp Oncol (Pozn) 2018;22:38-40.
20. Chen R, Xu X, Qian Z, et al. The biological functions and clinical applications of exosomes in lung cancer. Cell Mol Life Sci 2019;76:4613-33.
21. Hu C, Meiners S, Lukas C, Stathopoulos GT, Chen J. Role of exosomal microRNAs in lung cancer biology and clinical applications. Cell Prolif 2020;53:e12828.
22. Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol 2009;183:3720-30.
23. Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018;560:382-6.
24. Poggio M, Hu T, Pai CC, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019;177:414-27.e13.
25. Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res 2005;11:1010-20.
26. Abusamra AJ, Zhong Z, Zheng X, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis 2005;35:169-73.
27. Czystowska M, Han J, Szczepanski MJ, et al. IRX-2, a novel immunotherapeutic, protects human T cells from tumor-induced cell death. Cell Death Differ 2009;16:708-18.
28. Huang SH, Li Y, Zhang J, Rong J, Ye S. Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Invest 2013;31:330-5.
29. Yin Y, Cai X, Chen X, et al. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res 2014;24:1164-80.
30. Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One 2010;5:e11469.
31. Liu B, Zhang R, Zhu Y, Hao R. Exosome-derived microRNA-433 inhibits tumorigenesis through incremental infiltration of CD4 and CD8 cells in non-small cell lung cancer. Oncol Lett 2021;22:607.
32. Fabbri M, Paone A, Calore F, et al. MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A 2012;109:E2110-6.
33. Marcus A, Gowen BG, Thompson TW, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol 2014;122:91-128.
34. Alipoor SD, Mortaz E, Varahram M, et al. The potential biomarkers and immunological effects of tumor-derived exosomes in lung cancer. Front Immunol 2018;9:819.
35. Berchem G, Noman MZ, Bosseler M, et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology 2016;5:e1062968.
36. Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans 2013;41:245-51.
37. Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol 2008;180:7249-58.
38. Ashiru O, Boutet P, Fernández-Messina L, et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res 2010;70:481-9.
39. Liu Y, Xiang X, Zhuang X, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol 2010;176:2490-9.
40. Deng W, Gowen BG, Zhang L, et al. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science 2015;348:136-9.
41. Filipazzi P, Bürdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol 2012;22:342-9.
42. Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica 2011;96:1302-9.
43. Kunigelis KE, Graner MW. The dichotomy of tumor exosomes (TEX) in cancer immunity: is it all in the ConTEXt? Vaccines (Basel) 2015;3:1019-51.
44. Hosseini R, Asef-Kabiri L, Yousefi H, et al. The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol Cancer 2021;20:83.
45. Ning Y, Shen K, Wu Q, et al. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol Lett 2018;199:36-43.
46. Ludwig S, Sharma P, Theodoraki MN, et al. Molecular and functional profiles of exosomes from HPV(+) and HPV(-) head and neck cancer cell lines. Front Oncol 2018;8:445.
47. Xiang X, Poliakov A, Liu C, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 2009;124:2621-33.
48. Chalmin F, Ladoire S, Mignot G, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 2010;120:457-71.
49. Mignot G, Chalmin F, Ladoire S, Rébé C, Ghiringhelli F. Tumor exosome-mediated MDSC activation. Am J Pathol 2011;178:1403-4; author reply 1404-5.
50. Xiang X, Liu Y, Zhuang X, et al. TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol 2010;177:1606-10.
51. Zhang X, Li F, Tang Y, et al. miR-21a in exosomes from Lewis lung carcinoma cells accelerates tumor growth through targeting PDCD4 to enhance expansion of myeloid-derived suppressor cells. Oncogene 2020;39:6354-69.
52. Hsu YL, Hung JY, Chang WA, et al. Hypoxic lung-cancer-derived extracellular vesicle microRNA-103a increases the oncogenic effects of macrophages by targeting PTEN. Mol Ther 2018;26:568-81.
53. Pritchard A, Tousif S, Wang Y, et al. Lung tumor cell-derived exosomes promote M2 macrophage polarization. Cells 2020;9:1303.
54. Chen T, Liu Y, Li C, et al. Tumor-derived exosomal circFARSA mediates M2 macrophage polarization via the PTEN/PI3K/AKT pathway to promote non-small cell lung cancer metastasis. Cancer Treat Res Commun 2021;28:100412.
55. Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer 2019;1871:455-68.
56. Whiteside TL, Diergaarde B, Hong CS. Tumor-derived exosomes (TEX) and their role in immuno-oncology. Int J Mol Sci 2021;22:6234.
57. Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 2011;33:419-40.
58. Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol 2015;40:72-81.
59. Gastpar R, Gehrmann M, Bausero MA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 2005;65:5238-47.
60. Vega VL, Rodríguez-Silva M, Frey T, et al. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol 2008;180:4299-307.
61. Li XB, Zhang ZR, Schluesener HJ, Xu SQ. Role of exosomes in immune regulation. J Cell Mol Med 2006;10:364-75.
62. Liu J, Luo R, Wang J, et al. Tumor cell-derived exosomal miR-770 inhibits M2 macrophage polarization via targeting MAP3K1 to Inhibit the invasion of non-small cell lung cancer cells. Front Cell Dev Biol 2021;9:679658.
63. Jiang L, Gu Y, Du Y, Liu J. Exosomes: diagnostic biomarkers and therapeutic delivery vehicles for cancer. Mol Pharm 2019;16:3333-49.
64. Nedaeinia R, Manian M, Jazayeri MH, et al. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther 2017;24:48-56.
65. Shan Z, Wang H, Zhang Y, Min W. The role of tumor-derived exosomes in the abscopal effect and immunotherapy. Life (Basel) 2021;11:381.
66. Jakobsen KR, Paulsen BS, Bæk R, Varming K, Sorensen BS, Jørgensen MM. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles 2015;4:26659.
67. Vykoukal J, Sun N, Aguilar-Bonavides C, et al. Plasma-derived extracellular vesicle proteins as a source of biomarkers for lung adenocarcinoma. Oncotarget 2017;8:95466-80.
68. Niu L, Song X, Wang N, Xue L, Song X, Xie L. Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci 2019;110:433-42.
69. Gao J, Qiu X, Li X, et al. Expression profiles and clinical value of plasma exosomal Tim-3 and Galectin-9 in non-small cell lung cancer. Biochem Biophys Res Commun 2018;498:409-15.
70. Li Y, Zhang Y, Qiu F, Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis 2011;32:1976-83.
71. Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer 2009;10:42-6.
72. Jin X, Chen Y, Chen H, et al. Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for early-stage non-small cell lung cancer using next-generation sequencing. Clin Cancer Res 2017;23:5311-9.
73. Xia J, Luo M, Dai L, Wang L, Wang L, Zhu J. Serum exosomal microRNAs as predictive markers for EGFR mutations in non-small-cell lung cancer. J Clin Lab Anal 2021;35:e23743.
74. Zhang ZJ, Song XG, Xie L, et al. Circulating serum exosomal miR-20b-5p and miR-3187-5p as efficient diagnostic biomarkers for early-stage non-small cell lung cancer. Exp Biol Med (Maywood) 2020;245:1428-36.
75. Grimolizzi F, Monaco F, Leoni F, et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci Rep 2017;7:15277.
76. Tamiya H, Mitani A, Saito A, et al. Exosomal MicroRNA expression profiling in patients with lung adenocarcinoma-associated malignant pleural effusion. Anticancer Res 2018;38:6707-14.
77. Hydbring P, De Petris L, Zhang Y, et al. Exosomal RNA-profiling of pleural effusions identifies adenocarcinoma patients through elevated miR-200 and LCN2 expression. Lung Cancer 2018;124:45-52.
78. Dejima H, Iinuma H, Kanaoka R, Matsutani N, Kawamura M. Exosomal microRNA in plasma as a non-invasive biomarker for the recurrence of non-small cell lung cancer. Oncol Lett 2017;13:1256-63.
79. Zhang L, Hao C, Zhai R, et al. Downregulation of exosomal let-7a-5p in dust exposed- workers contributes to lung cancer development. Respir Res 2018;19:235.
80. Luo R, Liu H, Chen J. Reduced circulating exosomal miR-382 predicts unfavorable outcome in non-small cell lung cancer. Int J Clin Exp Pathol 2021;14:469-74.
81. Zhang R, Xia Y, Wang Z, et al. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem Biophys Res Commun 2017;490:406-14.
82. Dong Q, Dong L, Liu S, Kong Y, Zhang M, Wang X. Tumor-derived exosomal eIF4E as a biomarker for survival prediction in patients with non-small cell lung cancer. Med Sci Monit 2020;26:e923210.
83. Xu X, Tao R, Sun L, Ji X. Exosome-transferred hsa_circ_0014235 promotes DDP chemoresistance and deteriorates the development of non-small cell lung cancer by mediating the miR-520a-5p/CDK4 pathway. Cancer Cell Int 2020;20:552.
84. Song Z, Jia G, Ma P, Cang S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci 2021;276:119399.
85. Li M, Shan W, Hua Y, et al. Exosomal miR-92b-3p promotes chemoresistance of small cell lung cancer through the PTEN/AKT pathway. Front Cell Dev Biol 2021;9:661602.
86. Dinh TK, Fendler W, Chałubińska-Fendler J, et al. Circulating miR-29a and miR-150 correlate with delivered dose during thoracic radiation therapy for non-small cell lung cancer. Radiat Oncol 2016;11:61.
87. Janpipatkul K, Trachu N, Watcharenwong P, et al. Exosomal microRNAs as potential biomarkers for osimertinib resistance of non-small cell lung cancer patients. Cancer Biomark 2021;31:281-94.
88. Hisakane K, Seike M, Sugano T, et al. Exosome-derived miR-210 involved in resistance to osimertinib and epithelial-mesenchymal transition in EGFR mutant non-small cell lung cancer cells. Thorac Cancer 2021;12:1690-8.
89. Yang B, Teng F, Chang L, et al. Tumor-derived exosomal circRNA_102481 contributes to EGFR-TKIs resistance via the miR-30a-5p/ROR1 axis in non-small cell lung cancer. Aging (Albany NY) 2021;13:13264-86.
90. Peng XX, Yu R, Wu X, et al. Correlation of plasma exosomal microRNAs with the efficacy of immunotherapy in EGFR/ALK wild-type advanced non-small cell lung cancer. J Immunother Cancer 2020;8:e000376.
91. Battke C, Ruiss R, Welsch U, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother 2011;60:639-48.
92. Meehan K, Vella LJ. The contribution of tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin Lab Sci 2016;53:121-31.
93. Kim DH, Kim H, Choi YJ, et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med 2019;51:1-13.
94. Pitt JM, Charrier M, Viaud S, et al. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol 2014;193:1006-11.
95. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release 2015;219:396-405.
96. van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 2012;64:676-705.
97. Wang J, Wang L, Lin Z, Tao L, Chen M. More efficient induction of antitumor T cell immunity by exosomes from CD40L gene-modified lung tumor cells. Mol Med Rep 2014;9:125-31.
98. Li W, Mu D, Tian F, et al. Exosomes derived from Rab27aoverexpressing tumor cells elicit efficient induction of antitumor immunity. Mol Med Rep 2013;8:1876-82.
99. Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996;183:1161-72.
100. Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998;4:594-600.
101. Lee EY, Park KS, Yoon YJ, et al. Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis. PLoS One 2012;7:e33330.
102. Marton A, Vizler C, Kusz E, et al. Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol Lett 2012;148:34-8.
103. Gu X, Erb U, Büchler MW, Zöller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer 2015;136:E74-84.
104. Wang C, Huang X, Wu Y, Wang J, Li F, Guo G. Tumor cell-associated exosomes robustly elicit anti-tumor immune responses through modulating dendritic cell vaccines in lung tumor. Int J Biol Sci 2020;16:633-43.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Wu J, Li S, Zhang P. Tumor-derived exosomes: immune properties and clinical application in lung cancer. Cancer Drug Resist 2022;5:102-13. http://dx.doi.org/10.20517/cdr.2021.99
AMA Style
Wu J, Li S, Zhang P. Tumor-derived exosomes: immune properties and clinical application in lung cancer. Cancer Drug Resistance. 2022; 5(1): 102-13. http://dx.doi.org/10.20517/cdr.2021.99
Chicago/Turabian Style
Wu, Jing, Suyao Li, Pengfei Zhang. 2022. "Tumor-derived exosomes: immune properties and clinical application in lung cancer" Cancer Drug Resistance. 5, no.1: 102-13. http://dx.doi.org/10.20517/cdr.2021.99
ACS Style
Wu, J.; Li S.; Zhang P. Tumor-derived exosomes: immune properties and clinical application in lung cancer. Cancer Drug Resist. 2022, 5, 102-13. http://dx.doi.org/10.20517/cdr.2021.99
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 6 clicks
Cite This Article 6 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.