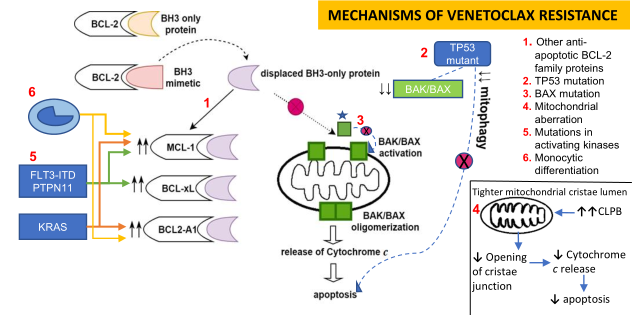
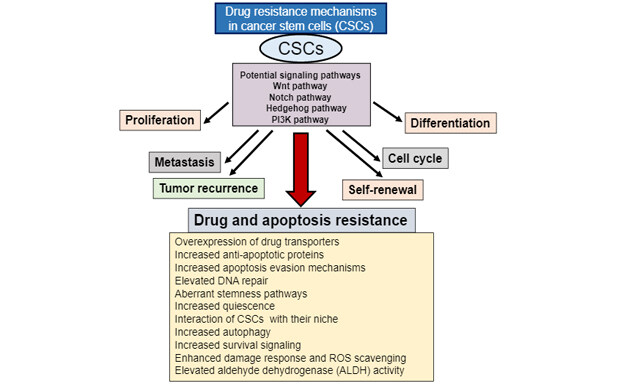
Cancer Drug Resistance
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Data
1535
Authors
1021
Reviewers
2018
Published Since
1,358,748
Article Views
401,305
Article Downloads
For Reviewers
For Readers
Add your e-mail address to receive forthcoming Issues of this journal:
Themed Collections
Resistance
Drug delivery
Nanomedicine
Tumor microenvironment
Immune checkpoint inhibitors
Target therapy
Immunotherapy
Radiotherapy
Ferroptosis
PARP inhibitors
MYC
ABC transporters
Autophagy
Metabolism
Mechanisms
Precision medicine
Extracellular vesicles
Stem cell
Biomarker
Ovarian cancer
Renal cell carcinoma
Prostate cancer
Breast cancer
Colorectal cancer
Myeloid leukemia
Non-small cell lung cancer
Pancreatic cancer
Head and neck cancer
Squamous cell carcinoma
Mitochondria
Volume 7
Volume 6, Issue 4
Volume 6, Issue 3
Volume 6, Issue 2
Volume 6, Issue 1
Volume 5, Issue 4
Volume 5, Issue 3
Volume 5, Issue 2
Volume 5, Issue 1
Volume 4, Issue 4
Volume 4, Issue 3
Volume 4, Issue 2
Volume 4, Issue 1
Volume 3, Issue 4
Volume 3, Issue 3
Volume 3, Issue 2
Volume 3, Issue 1
Volume 2, Issue 4
Volume 2, Issue 3
Volume 2, Issue 2
Volume 2, Issue 1
Volume 1, Issue 4
Volume 1, Issue 3
Volume 1, Issue 2
Volume 1, Issue 1
Related Journals
Related Journals
Data
1535
Authors
1021
Reviewers
2018
Published Since
1,358,748
Article Views
401,305
Article Downloads