Tackling heterogeneity in treatment-resistant breast cancer using a broad-spectrum therapeutic approach
Abstract
Tumor heterogeneity can contribute to the development of therapeutic resistance in cancer, including advanced breast cancers. The object of the Halifax project was to identify new treatments that would address mechanisms of therapeutic resistance through tumor heterogeneity by uncovering combinations of therapeutics that could target the hallmarks of cancer rather than focusing on individual gene products. A taskforce of 180 cancer researchers, used molecular profiling to highlight key targets responsible for each of the hallmarks of cancer and then find existing therapeutic agents that could be used to reach those targets with limited toxicity. In many cases, natural health products and re-purposed pharmaceuticals were identified as potential agents. Hence, by combining the molecular profiling of tumors with therapeutics that target the hallmark features of cancer, the heterogeneity of advanced-stage breast cancers can be addressed.
Keywords
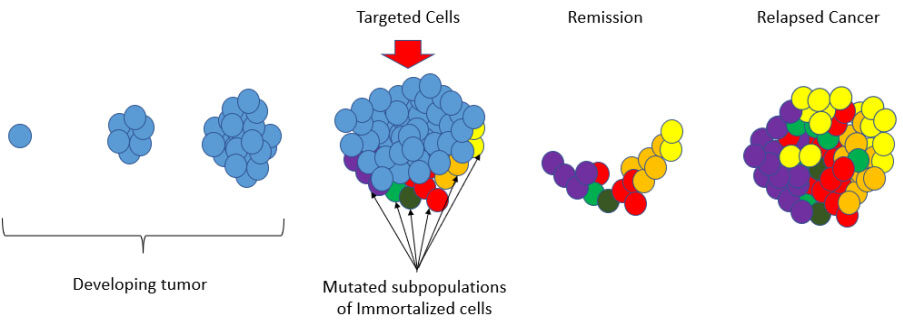
Breast cancer is a consequence of complex epigenetic and genetic alterations. The heterogeneity and evolution within breast cancers underpin tumor progression, as well as therapeutic resistance[1-3]. In recent years, significant efforts have focused on addressing therapeutic options that can address tumor heterogeneity in breast cancer[4-6]. This is particularly important in triple-negative breast cancers and metastatic breast cancers because many patients with advanced breast cancers will succumb to their disease as tumor heterogeneity gives rise to therapeutic resistance[7-9] [Figure 1].
Figure 1. Therapeutic resistance - Developing tumors begin with a single immortalized cell that may have a single therapeutic target that can act to stop those cells from replication. For example, Tamoxifen (TAM) is the most common therapy used for the treatment of estrogen receptor-positive (ER+) breast cancer and it is used successfully in many cases. However, there are advanced-stage breast cancers that are plagued with mutated subpopulations of immortalized cells and cancer stem cells that play a key role in breast cancer progression, and metastasis. In these cancers, a single targeted therapy may produce a remission by successfully arresting some of the immortalized cells that have been targeted. However, if the remaining subpopulations of cells are driven by different mechanisms and prove to be chemoresistant, they will persist during remission and ultimately produce a relapsed cancer that is fully refractory to the initial treatment or combination of treatments.
To address the challenge of therapeutic resistance and tumor heterogeneity, a group of 180 cancer researchers collaborated in “The Halifax Project” to consider combinations of agents that might be employed[10]. In this effort, twelve teams of researchers were organized around the Hallmarks of Cancer[11] and tasked to identify high-priority targets along with corresponding therapeutic agents that could reach those targets with limited toxicity. The hallmarks used are attributes ultimately found in most cancers (i.e., genomic instability, sustained proliferative signaling, tumor-promoting inflammation, evasion of anti-growth signaling, resistance to apoptosis, replicative immortality, dysregulated metabolism, immune system evasion, angiogenesis, tissue invasion and metastasis, and an accommodating tumor micro-environment). The overarching goal was to identify a significant number of agents that have limited to no toxicity, that might be combined to reach a multitude of key targets simultaneously.
Cancer is caused by an array of mutations and genomic events that coordinate to activate aberrant pathways. To address this complexity, we used the Hallmarks of Cancer as an organizing framework. We then focused on the development of a "broad-spectrum" methodology and therapeutic agents that could be combined using this approach. In particular, we focused on identifying natural health products (NHPs) and re-purposed pharmaceuticals, because both are readily available, often well tolerated, and broadly applicable to many cancers[10,12-22]. A detailed rationale for the methodology was provided and it was determined that it should be feasible from a safety standpoint and relatively inexpensive to implement[23].
In the table below [Table 1] we have provided a sampling of NHP with updated references to show how these agents act on key mechanisms and pathways across the hallmarks of cancer[10-22].
Aligning targets with the hallmarks of cancer
| Cancer hallmark | Examples of potential agents | Key mechanism or pathway |
| Genomic instability | Allyl Isothiocyanate[24] Chrysin[25] Plumbagin[26] | DNA damage and condensation DNA double-strand break repair DNA damage |
| Sustained proliferative signaling | Resveratrol[27] Perillyl alcohol[28] Artemisinin[29] | Cell cycle Cell cycle Cell cycle |
| Tumor-promoting Inflammation | Rosmarinic acid[30] Berberine[31] Curcumin[32] Punica granatum L[33] | NF3 kappaB-p53-caspase-3 pathways NLRP3 Inflammasome pathway Nuclear factor-κB (NF-κB) miRNA-27a and miRNA-155 |
| Evasion of anti-growth signaling | Deguelin[34] Luteolin[35] Withaferin A[36,37] Curcumin[38] | EGFR-p-AKT/c-Met p-ERK AKT/mTOR pathway Notch2 SLC1A5-mediated ferroptosis |
| Resistance to apoptosis | EGCG[39] Gossypol[40] Triptolide[41] Kaempferol[42] Berberine[43,44] | P53/Bcl-2 pathway miRNA expression of many apoptosis‑related genes p38/Erk/mTOR Bcl2 Bcl2 (and many other pathways) |
| Replicative immortality | Curcumin[45,46] Silibinin[46] Coumestrol[47] Diosmin[48] | Telomerase expression Telomerase expression Protein kinase CKII Senescence |
| Dysregulated metabolism | Resveratrol[49] Metformin[50] Baicalein[51] Carpesium abrotanoides L.[52] | 6-phosphofructo-1-kinase HIF-1alpha HIF-1alpha Glucose Metabolism and PKM2/HIF-1alpha axis |
| Immune system evasion | Astragalus polysaccharides[53] Cordycepin[54] Resveratrol[55] | Macrophage activation IL-2, TGF-β, IL-4 MICA/B and natural killer cells |
| Angiogenesis | Curcumin[56] EGCG[57] Melatonin[58,59] Resveratrol[60] | NF-κB pathway VEGF VEGF VEGF |
| Tissue invasion and metastasis | Diallyl trisulfides[61] Resveratrol[62] Anthocyanins[63] Cordycepin[64] | HIF-1alpha TGF-beta1 / Epithelial-Mesenchymal Transition FAK Hedgehog pathway |
| Tumor micro-environment | Resveratrol[65] Sulforaphane[66,67] | Macrophage polarization Adipose mesenchymal stem cells |
Some NHPs, such as curcumin and resveratrol, target multiple signaling networks and pathways simultaneously which is attractive molecular promiscuity[68,69]. The appropriate selection of NHPs can also offer synergies for chemotherapy and radiation therapy treatment. For example, curcumin, resveratrol, tocotrienol, garcinol and quercetin have a mechanism of action that increases chemosensitivity[70,71] and can reduce chemoresistance. While other NHPs, such as ellagic acid, diindolylmethane, and berberine, can increase radiation sensitivity[72-74].
Other NHPs, such as gingerol and curcumin can also protect normal (healthy) cells from adverse toxicity from cytotoxic agents[75,76]. Thus, NHPs have many features that may designate them particularly suited as agents that might be used in situations where tumor heterogeneity has resulted in chemoresistance.
In particular, for breast cancer, the presence of subpopulations of cancer stem cells (CSCs) is known to be one cause of chemo-resistance and ultimately contributes to therapeutic relapse[77]. Notably, there are specific NHPs such as sulforaphane, curcumin, genistein, resveratrol, lycopene, and epigallocatechin-3-gallate that have been shown to promote cell cycle arrest and apoptosis in triple-negative breast cancer cells and which have also been shown to inhibit important CSC pathways, such as NF-κB, PI3K/Akt/mTOR, Notch 1, Wnt/β-catenin, and YAP[78].
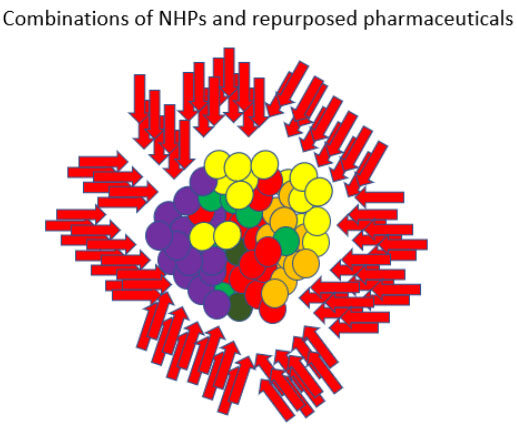
In addition to NHPs, there are also many existing pharmaceuticals that could provide additional targeting options. Numerous commonly prescribed non-oncology drugs possess multi-targeted anti-cancer effects. Pharmaceuticals already on the market have significant safety records and robust drug-drug interaction data compared to natural products and several researchers have looked at the effects of existing pharmaceuticals as it relates to relapse. Retsky (2012, 2020), for example, observed that the perioperative use of the NSAID analgesic ketorolac appears to reduce early relapse following mastectomy in breast cancer[79,80]. Hence, the use of both NHPs and repurposed pharmaceuticals to reach a broad-spectrum of molecular targets could be useful in developing personalized treatment protocols[81] [Figure 2].
Figure 2. A broad-spectrum approach - In a broad-spectrum approach, heterogeneous subpopulations of chemoresistant immortalized cells are not targeted using a single targeted therapy or even a combination of 2-3 chemotherapy agents. Instead, a significant number of low toxicity agents are aimed at a multitude of key pathways/mechanisms simultaneously. Since most immortalized cells are driven by the pathways/mechanisms described in the Hallmarks of Cancer framework[11], this approach increases the chance that a significant number of synergistic effects will be produced (i.e., since each affected cell will potentially be acted on in a multitude of ways).
Although our proposed approach has many potential advantages, there are challenges to conducting validating clinical studies. First, there is a shortage of funding for this type of initiative due to lack of patentability, manufacturing difficulties, contamination, and lack of product consistency[82]. Second, the use of NHPs among cancer patients is quite common. In fact, many patients who use them do not share the details with their physicians because they feel their physicians are not knowledgeable or will be indifferent or negative toward their use[83,84]. Finally, NHPs have yet to be approved by the FDA and although many NHPs are available as supplements and generally well-tolerated over an extended duration, the clinical evidence for these agents is often weak or non-existent.
One example of how NHPs can be used to improve the treatment of breast cancer is that NHPs have been shown to combine with Tamoxifen synergistically in inhibition of tumor cell growth, improved Tamoxifen sensitivity and reduction of Tamoxifen side effects[85]. However, some NHPs showed estrogen-like activity, which could reduce the effect of Tamoxifen, underscoring the need for a detailed analysis of any protocol that combines a multitude of agents[85]. They did find that some NHPs (e.g., morin, silybin, epigallocatechin gallate, myricetin, baicalein, curcumin, kaempferol, and quercetin) helped to increase the bioavailability of Tamoxifen in vivo. These promising observations suggest that NHPs with Tamoxifen are worthy of clinical studies.
Some NHPs are used to support cancer therapy by clinicians who practice integrative oncology; however, these physicians are typically less familiar with the molecular mechanisms of cancer signaling[86]. Instead, integrative oncology mainly focuses on the treatment of cancer-related symptoms such as acupuncture for nausea, exercise for sleep, and anxiety[86]. Indeed, a survey of clinics in Washington State showed that more than 72 oral or topical, nutritional, botanical, fungal and bacterial-based medicines had been used during the first year of care of the female breast cancer patients studied (n = 324)[87]. Since most of these agents are not aimed at the molecular mechanisms of cancer, the use of NHPs for these purposes would typically not include the type of analysis that would be needed to target tumor heterogeneity.
We highlight this approach as an important avenue that should be investigated further because the idea of reaching many key targets simultaneously makes sense given what is now known about the biology of cancer. Importantly, this is not something that has been attempted previously. Clinical trials of NHPs that have been undertaken typically involve single agents or limited combinations of agents at best. We speculate the use of combinations of NHPs that are able to hit multiple targets is most likely to be clinically effective.
The goal should be to provide a clinical treatment protocol that makes a rational utilization of the evidence base. If this approach is to work, future efforts utilizing NHPs and repurposed pharmaceuticals will require clinical studies involving unique combinations of dozens of agents in protocols that are tailored/personalized for each patient. The agents that are used will therefore need to be carefully considered for potential interactions, and some of these agents may have shown limited or no activity when used individually. Until there is clinical research that fully explores the synergies that can be produced when a significant number of pathways are targeted simultaneously, the true potential of combining these actions all at once will simply not be known.
Finally, we acknowledge that what we are proposing would require a change from phased clinical trials. A case series or a cohort study might be a more appropriate means to document the results of experimental efforts of this nature[88], since each patient will require an individualized protocol. We do believe that such an approach may be able to help address the challenges of therapeutic resistance that emerge in many breast cancers.
DECLARATIONS
Authors’ contributionsAll authors contributed equally.
Availability of data and materialsNot applicable.
Financial support and sponsorshipLowe L was not supported financially or sponsored, Felsher DW is supported by multiple grants from the National Institute of Health, LaValley JW was not supported financially or sponsored.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Mavrommati I, Johnson F, Echeverria GV, Natrajan R. Subclonal heterogeneity and evolution in breast cancer. NPJ Breast Cancer 2021;7:155.
2. Yang R, Li Y, Wang H, Qin T, Yin X, Ma X. Therapeutic progress and challenges for triple negative breast cancer: targeted therapy and immunotherapy. Mol Biomed 2022;3:8.
3. Turner KM, Yeo SK, Holm TM, Shaughnessy E, Guan JL. Heterogeneity within molecular subtypes of breast cancer. Am J Physiol Cell Physiol 2021;321:C343-54.
4. Blucher AS, Mills GB, Tsang YH. Precision oncology for breast cancer through clinical trials. Clin Exp Metastasis 2022;39:71-8.
5. Zambelli A, Sgarra R, De Sanctis R, Agostinetto E, Santoro A, Manfioletti G. Heterogeneity of triple-negative breast cancer: understanding the Daedalian labyrinth and how it could reveal new drug targets. Expert Opin Ther Targets 2022;26:557-73.
6. Pasha N, Turner NC. Understanding and overcoming tumor heterogeneity in metastatic breast cancer treatment. Nat Cancer 2021;2:680-92.
7. Ferrari P, Scatena C, Ghilli M, Bargagna I, Lorenzini G, Nicolini A. Molecular mechanisms, biomarkers and emerging therapies for chemotherapy resistant TNBC. Int J Mol Sci 2022;23:1665.
8. Li A, Keck JM, Parmar S, et al. Characterizing advanced breast cancer heterogeneity and treatment resistance through serial biopsies and comprehensive analytics. NPJ Precis Oncol 2021;5:28.
9. Zong Y, Pegram M. Research advances and new challenges in overcoming triple-negative breast cancer. Cancer Drug Resist 2021;4:517-42.
10. Bishayee A, Block K. A broad-spectrum integrative design for cancer prevention and therapy: The challenge ahead. Semin Cancer Biol 2015;35 Suppl:S1-4.
12. Ferguson LR, Chen H, Collins AR, et al. Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol 2015;35 Suppl:S5-S24.
13. Feitelson MA, Arzumanyan A, Kulathinal RJ, et al. Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin Cancer Biol 2015;35 Suppl:S25-54.
14. Amin ARMR, Karpowicz PA, Carey TE, et al. Evasion of anti-growth signaling: a key step in tumorigenesis and potential target for treatment and prophylaxis by natural compounds. Semin Cancer Biol 2015;35 Suppl:S55-77.
15. Mohammad RM, Muqbil I, Lowe L, et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol 2015;35 Suppl:S78-S103.
16. Yaswen P, MacKenzie KL, Keith WN, et al. Therapeutic targeting of replicative immortality. Semin Cancer Biol 2015;35 Suppl:S104-28.
17. Hirschey MD, DeBerardinis RJ, Diehl AME, et al. Target Validation Team. Dysregulated metabolism contributes to oncogenesis. Semin Cancer Biol 2015;35 Suppl:S129-50.
18. Samadi AK, Bilsland A, Georgakilas AG, et al. A multi-targeted approach to suppress tumor-promoting inflammation. Semin Cancer Biol 2015;35 Suppl:S151-84.
19. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015;35 Suppl:S185-98.
20. Casey SC, Amedei A, Aquilano K, et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol 2015;35 Suppl:S199-223.
21. Wang Z, Dabrosin C, Yin X, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol 2015;35 Suppl:S224-43.
22. Jiang WG, Sanders AJ, Katoh M, et al. Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin Cancer Biol 2015;35 Suppl:S244-75.
23. Block KI, Gyllenhaal C, Lowe L, et al. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin Cancer Biol 2015;35 Suppl:S276-304.
24. Liao CL, Peng SF, Chen JC, et al. Allyl isothiocyanate induces DNA damage and impairs DNA repair in human breast cancer MCF-7 cells. Anticancer Res 2021;41:4343-51.
25. Geng A, Xu S, Yao Y, et al. Chrysin impairs genomic stability by suppressing DNA double-strand break repair in breast cancer cells. Cell Cycle 2022;21:379-91.
26. Sameni S, Hande MP. Plumbagin triggers DNA damage response, telomere dysfunction and genome instability of human breast cancer cells. Biomed Pharmacother 2016;82:256-68.
27. Kowsari H, Davoodvandi A, Dashti F, et al. Resveratrol in cancer treatment with a focus on breast cancer. Curr Mol Pharmacol 2022; doi: 10.2174/1874467215666220616145216.
28. Yuri T, Danbara N, Tsujita-kyutoku M, et al. Perillyl alcohol inhibits human breast cancer cell growth in vitro and in vivo. Breast Cancer Res Treat 2004;84:251-60.
29. Guan X, Guan Y. Artemisinin induces selective and potent anticancer effects in drug resistant breast cancer cells by inducing cellular apoptosis and autophagy and G2/M cell cycle arrest. J BUON 2020;25:1330-6.
30. Mahmoud MA, Okda TM, Omran GA, Abd-Alhaseeb MM. Rosmarinic acid suppresses inflammation, angiogenesis, and improves paclitaxel induced apoptosis in a breast cancer model via NF3 κB-p53-caspase-3 pathways modulation. J Appl Biomed 2021;19:202-9.
31. Yao M, Fan X, Yuan B, et al. Berberine inhibits NLRP3 Inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complement Altern Med 2019;19:216.
32. Song X, Zhang M, Dai E, Luo Y. Molecular targets of curcumin in breast cancer (Review). Mol Med Rep 2019;19:23-9.
33. Banerjee N, Talcott S, Safe S, Mertens-Talcott SU. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: potential role of miRNA-27a and miRNA-155 in cell survival and inflammation. Breast Cancer Res Treat 2012;136:21-34.
34. Mehta R, Katta H, Alimirah F, et al. Deguelin action involves c-Met and EGFR signaling pathways in triple negative breast cancer cells. PLoS One 2013;8:e65113.
35. Wu HT, Lin J, Liu YE, et al. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021;81:153437.
36. Kim SH, Hahm ER, Arlotti JA, et al. Withaferin a inhibits in vivo growth of breast cancer cells accelerated by Notch2 knockdown. Breast Cancer Res Treat 2016;157:41-54.
37. Hahm ER, Kim SH, Singh KB, Singh K, Singh SV. A comprehensive review and perspective on anticancer mechanisms of withaferin a in breast cancer. Cancer Prev Res (Phila) 2020;13:721-34.
38. Cao X, Li Y, Wang Y, et al. Curcumin suppresses tumorigenesis by ferroptosis in breast cancer. PLoS One 2022;17:e0261370.
39. Huang CY, Han Z, Li X, Xie HH, Zhu SS. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol Lett 2017;14:3623-7.
40. Messeha SS, Zarmouh NO, Mendonca P, Alwagdani H, Cotton C, Soliman KFA. Effects of gossypol on apoptosis-related gene expression in racially distinct triple-negative breast cancer cells. Oncol Rep 2019;42:467-78.
41. Gao H, Zhang Y, Dong L, et al. Triptolide induces autophagy and apoptosis through ERK activation in human breast cancer MCF-7 cells. Exp Ther Med 2018;15:3413-9.
43. Khalki L, Maire V, Dubois T, Zyad A. Berberine impairs the survival of triple negative breast cancer cells: cellular and molecular analyses. Molecules 2020;25:506.
44. Zhu Y, Xie N, Chai Y, et al. Apoptosis induction, a sharp edge of berberine to exert anti-cancer effects, focus on breast, lung, and liver cancer. Front Pharmacol 2022;13:803717.
45. Ávila-Gálvez MÁ, González-Sarrías A, Martínez-Díaz F, et al. Disposition of dietary polyphenols in breast cancer patients’ tumors, and their associated anticancer activity: the particular case of curcumin. Mol Nutr Food Res 2021;65:e2100163.
46. Nasiri M, Zarghami N, Koshki KN, et al. Curcumin and silibinin inhibit telomerase expression in T47D human breast cancer cells. Asian Pac J Cancer Prev 2013;14:3449-53.
47. Lee YH, Yuk HJ, Park KH, Bae YS. Coumestrol induces senescence through protein kinase CKII inhibition-mediated reactive oxygen species production in human breast cancer and colon cancer cells. Food Chem 2013;141:381-8.
48. Lewinska A, Adamczyk-Grochala J, Kwasniewicz E, Deregowska A, Wnuk M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol Lett 2017;265:117-30.
49. Gomez LS, Zancan P, Marcondes MC, et al. Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie 2013;95:1336-43.
50. Shao S, Zhao L, An G, et al. Metformin suppresses HIF-1α expression in cancer-associated fibroblasts to prevent tumor-stromal cross talk in breast cancer. FASEB J 2020;34:10860-70.
51. Chen Y, Zhang J, Zhang M, et al. Baicalein resensitizes tamoxifen-resistant breast cancer cells by reducing aerobic glycolysis and reversing mitochondrial dysfunction via inhibition of hypoxia-inducible factor-1α. Clin Transl Med 2021;11:e577.
52. Chai XX, Le YF, Wang JC, et al. Carpesium abrotanoides (L.) root as a potential source of natural anticancer compounds: targeting glucose metabolism and PKM2/HIF-1α axis of breast cancer cells. J Food Sci 2019;84:3825-32.
53. Li W, Song K, Wang S, et al. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater Sci Eng C Mater Biol Appl 2019;98:685-95.
54. Jeong MH, Lee CM, Lee SW, et al. Cordycepin-enriched Cordyceps militaris induces immunomodulation and tumor growth delay in mouse-derived breast cancer. Oncol Rep 2013;30:1996-2002.
55. Pan J, Shen J, Si W, et al. Resveratrol promotes MICA/B expression and natural killer cell lysis of breast cancer cells by suppressing c-Myc/miR-17 pathway. Oncotarget 2017;8:65743-58.
56. Bimonte S, Barbieri A, Palma G, et al. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. Biomed Res Int 2015;2015:878134.
57. Gu JW, Makey KL, Tucker KB, et al. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc Cell 2013;5:9.
58. Karadas AK, Dilmac S, Aytac G, Tanriover G. Melatonin decreases metastasis, primary tumor growth and angiogenesis in a mice model of breast cancer. Hum Exp Toxicol 2021;40:1545-57.
59. Alvarez-García V, González A, Alonso-González C, Martínez-Campa C, Cos S. Regulation of vascular endothelial growth factor by melatonin in human breast cancer cells. J Pineal Res 2013;54:373-80.
60. Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett 2006;231:113-22.
61. Wei Z, Shan Y, Tao L, et al. Diallyl trisulfides, a natural histone deacetylase inhibitor, attenuate HIF-1α synthesis, and decreases breast cancer metastasis. Mol Carcinog 2017;56:2317-31.
62. Sun Y, Zhou QM, Lu YY, et al. Resveratrol inhibits the migration and metastasis of MDA-MB-231 human breast cancer by reversing TGF-β1-induced epithelial-mesenchymal transition. Molecules 2019;24:1131.
63. Zhou J, Zhu YF, Chen XY, et al. Black rice-derived anthocyanins inhibit HER-2-positive breast cancer epithelial-mesenchymal transition-mediated metastasis in vitro by suppressing FAK signaling. Int J Mol Med 2017;40:1649-56.
64. Liu C, Qi M, Li L, Yuan Y, Wu X, Fu J. Natural cordycepin induces apoptosis and suppresses metastasis in breast cancer cells by inhibiting the Hedgehog pathway. Food Funct 2020;11:2107-16.
65. Cheuk IW, Chen J, Siu M, et al. Resveratrol enhanced chemosensitivity by reversing macrophage polarization in breast cancer. Clin Transl Oncol 2022;24:854-63.
66. Li Q, Xia J, Yao Y, Gong DW, Shi H, Zhou Q. Sulforaphane inhibits mammary adipogenesis by targeting adipose mesenchymal stem cells. Breast Cancer Res Treat 2013;141:317-24.
67. Rong Y, Huang L, Yi K, et al. Co-administration of sulforaphane and doxorubicin attenuates breast cancer growth by preventing the accumulation of myeloid-derived suppressor cells. Cancer Lett 2020;493:189-96.
68. Farghadani R, Naidu R. Curcumin as an enhancer of therapeutic efficiency of chemotherapy drugs in breast cancer. Int J Mol Sci 2022;23:2144.
69. Ávila-Gálvez MÁ, Giménez-Bastida JA, Espín JC, González-Sarrías A. Dietary phenolics against breast cancer. A critical evidence-based review and future perspectives. Int J Mol Sci 2020;21:5718.
70. Monisha J, Padmavathi G, Roy NK, et al. NF-κB blockers gifted by mother nature: prospectives in cancer cell chemosensitization. Curr Pharm Des 2016;22:4173-200.
71. Khatoon E, Banik K, Harsha C, et al. Phytochemicals in cancer cell chemosensitization: current knowledge and future perspectives. Semin Cancer Biol 2022;80:306-39.
72. Ahire V, Kumar A, Mishra KP, Kulkarni G. Ellagic acid enhances apoptotic sensitivity of breast cancer cells to γ-radiation. Nutr Cancer 2017;69:904-10.
73. Wang W, Lv M, Huangfu C, Wang F, Zhang J. 3,3’-Diindolylmethane: a promising sensitizer of γ-irradiation. Biomed Res Int 2015;2015:465105.
74. Wang J, Liu Q, Yang Q. Radiosensitization effects of berberine on human breast cancer cells. Int J Mol Med 2012;30:1166-72.
75. Al-Abbasi FA, Alghamdi EA, Baghdadi MA, et al. Gingerol synergizes the cytotoxic effects of doxorubicin against liver cancer cells and protects from its vascular toxicity. Molecules 2016;21:886.
76. Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer 2010;62:919-30.
77. Angelis ML, Francescangeli F, Zeuner A. Breast cancer stem cells as drivers of tumor chemoresistance, dormancy and relapse: new challenges and therapeutic opportunities. Cancers (Basel) 2019;11:1569.
78. Ke DYJ, El-Sahli S, Wang L. The potential of natural products in the treatment of triple-negative breast cancer. Curr Cancer Drug Targets 2022;22:388-403.
79. Retsky M, Demicheli R, Hrushesky WJ, et al. Promising development from translational or perhaps anti-translational research in breast cancer. Clin Transl Med 2012;1:17.
80. Retsky M, Demicheli R, Hrushesky W, et al. Breast cancer and the black swan. Ecancermedicalscience 2020;14:1050.
81. Deng X, Nakamura Y. Cancer precision medicine: from cancer screening to drug selection and personalized immunotherapy. Trends Pharmacol Sci 2017;38:15-24.
82. Paller CJ, Denmeade SR, Carducci MA. Challenges of conducting clinical trials of natural products to combat cancer. Clin Adv Hematol Oncol 2016;14:447-55.
84. Le TQ, Smith L, Harnett J. A systematic review - biologically-based complementary medicine use by people living with cancer - is a more clearly defined role for the pharmacist required? Res Social Adm Pharm 2017;13:1037-44.
85. Yen C, Zhao F, Yu Z, Zhu X, Li CG. Interactions between natural products and tamoxifen in breast cancer: a comprehensive literature review. Front Pharmacol 2022;13:847113.
86. Lopez G, McQuade J, Cohen L, et al. Integrative oncology physician consultations at a comprehensive cancer center: analysis of demographic, clinical and patient reported outcomes. J Cancer 2017;8:395-402.
87. Standish LJ, Dowd F, Sweet E, et al. Breast cancer integrative oncology care and its costs. Integr Cancer Ther 2017;16:85-95.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Lowe L, LaValley JW, Felsher DW. Tackling heterogeneity in treatment-resistant breast cancer using a broad-spectrum therapeutic approach. Cancer Drug Resist 2022;5:917-25. http://dx.doi.org/10.20517/cdr.2022.40
AMA Style
Lowe L, LaValley JW, Felsher DW. Tackling heterogeneity in treatment-resistant breast cancer using a broad-spectrum therapeutic approach. Cancer Drug Resistance. 2022; 5(4): 917-25. http://dx.doi.org/10.20517/cdr.2022.40
Chicago/Turabian Style
Lowe, Leroy, J. William LaValley, Dean W. Felsher. 2022. "Tackling heterogeneity in treatment-resistant breast cancer using a broad-spectrum therapeutic approach" Cancer Drug Resistance. 5, no.4: 917-25. http://dx.doi.org/10.20517/cdr.2022.40
ACS Style
Lowe, L.; LaValley JW.; Felsher DW. Tackling heterogeneity in treatment-resistant breast cancer using a broad-spectrum therapeutic approach. Cancer Drug Resist. 2022, 5, 917-25. http://dx.doi.org/10.20517/cdr.2022.40
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 10 clicks
Cite This Article 10 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.