GD2+ cancer stem cells in triple-negative breast cancer: mechanisms of resistance to breast cancer therapies
Abstract
Research has led to the development of tailored treatment options for different cancers in different patients. Despite some treatments being able to provide remarkable responses, nearly all current treatments encounter the same issue: resistance. Here, we discuss our experiences with how breast cancers resist therapies. The focus of our discussion revolves around the cancer stem cell subpopulation and their mechanisms for resistance.
Keywords
INTRODUCTION
In 2021, breast cancer became the most common globally diagnosed cancer, accounting for 12% of all annual cases, with over 40,000 American women projected to die of breast cancer in 2022[1]. Despite advancing therapies, treatment resistance has been shown to be responsible for up to 90% of cancer-related deaths[2,3]. The purpose of this perspective is to share our group’s cumulative experiences in researching breast cancer and the mechanisms of treatment resistance. To do this, we discuss literature and findings from our group and others. The discussions focus on the triple-negative breast cancer (TNBC) subtype and the cancer stem cell (CSC) subpopulation. Furthermore, we will place emphasis on the CSC biomarker GD2 due to its novelty and integral role in our research. We hope that our text will promote discussions and encourage other research groups to incorporate our understanding and findings into their projects.
Breast cancer subtypes: TNBC and hormone receptor therapies
Breast cancer is a complex malignancy that can be molecularly characterized into subtypes with unique disease progressions, treatment regimens, and prognoses[4]. Subtyping is based on the tumor’s hormone receptor status and can be grouped as follows: Luminal A (ER+, PR+, HER2-, KI67-), Luminal B (ER+, PR+, HER2±, KI67+), HER2 overexpression (ER-, PR-, HER2+), and Basal/Triple Negative (ER-, PR-, HER2-)[5]. Although TNBC can be further classified (basal-like 1, basal-like 2, immunomodulatory, mesenchymal, mesenchymal stem-like, and luminal androgen receptor), the overall characteristics are a highly invasive, metastatic, and recurrent breast cancer subtype with high mortality rates and few treatment options[6,7]. TNBC’s lack of receptor expression allows it to resist current hormone receptor therapies such as tamoxifen, aromatase inhibitors, luteinizing hormone-releasing hormone agonists, Faslodex, and trastuzumab[8-10]. As a result, current treatments are limited to conventional therapeutics such as tumor resection, chemotherapy, and radiation therapies[11]. Despite initial responses, TNBC patients have a higher rate of distant recurrence with significantly worse 3-year survival probabilities post-neoadjuvant treatment compared to other breast cancer subtypes[12]. Therefore, identifying specific therapeutic targets for TNBC is imperative for improving treatment outcomes for patients.
Cancer stem cells: resisting chemo- and radiotherapies
Carcinogenesis, the transformation of healthy to cancerous cells, is due to an accumulation of mutations that results in uncontrolled replication and can often begin with a single cell. Despite their monoclonal origin, tumors are composed of a heterogeneous population of cells with only a small subpopulation possessing tumorigenic potential[13]. CSCs are a sub-subpopulation of tumors that possess the ability to self-renew, have increased tumorigenic potential, and increased mobility. Additionally, CSCs are innately resistant to radiotherapy and chemotherapies[14-16]. Although chemo- and radiotherapies may be initially successful in killing off most cells in a tumor, they confer a selection pressure for CSCs. This leads to the recurrence of a treatment-resistant tumor and an overall poorer outcome for TNBC patients in comparison to other breast cancer subtypes.
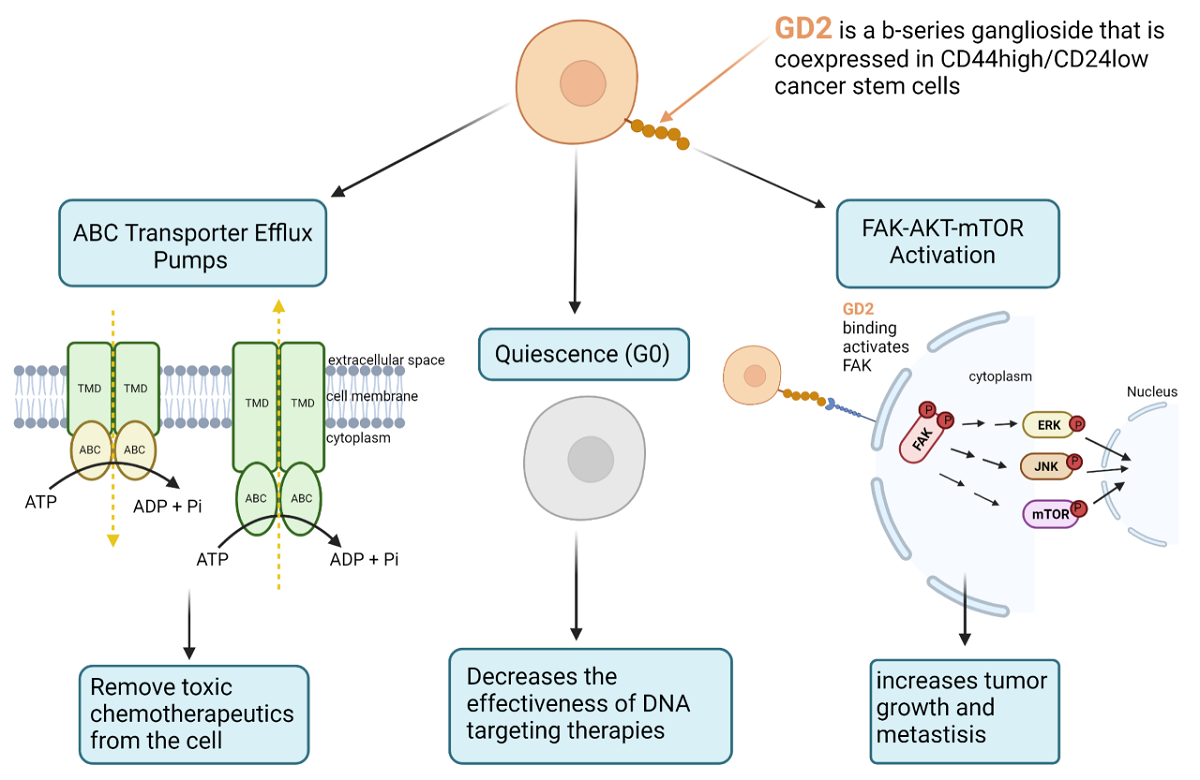
Chemotherapies and radiotherapy primarily affect rapidly dividing cells by interfering with DNA processing, leading to cellular apoptosis. Cancer stem cells have several mechanisms that allow them to resist and overcome the effects of chemotherapy. For example, CSCs have the ability to become quiescent in stressful conditions, hibernating in G0 and re-entering the cell cycle once conditions have improved. Malladi et al. identified SOX2 and SOX9 transcription factors producing DKK1 to inhibit WNT as the mechanism for quiescence in early-stage human lung and breast carcinoma cell lines[17]. This lack of cell division and, by association, DNA replication decreases the effectiveness of DNA targeting therapies such as chemo- and radiotherapy. In addition to becoming quiescent, CSCs can express ABC transporter proteins and efflux pumps that actively remove toxic compounds from within the cell. Das et al. were able to enhance the chemosensitivity of breast cancer stem cells by downregulating ABCG2 using nanoparticles[18]. Similarly, Sun et al. enhanced doxorubicin sensitivity, demonstrated by a 90% decrease in IC50, after 60% downregulation of ABCG2 was achieved by siRNA[19]. Although these studies demonstrate the significance of ABC transporters and efflux pumps in CSCs, it is important to note that varying levels of these proteins can be expressed in the non-stem population of the tumor as well.
Apoptosis is the natural cell death response to DNA damage. If quiescence and ABC transport proteins fail to prevent DNA damage from chemo- and radiotherapies, cancer cells will undergo apoptosis. This can be seen during the initial responsiveness of tumors to chemo- and radiotherapies. However, cancer stem cells have intrinsic mechanisms that allow them to avoid apoptosis. Takeda et al. identified a ROCK-survivin anti-apoptotic axis in pancreatic cancer stem cells and that inhibiting ROCK resulted in decreased survivin expression, leading to the sensitization of cells to gemcitabine[20]. Although there are more pathways associated with cancer stem cells and their resistance to therapies, such as Hippo/YAP1, Wnt/ß-catenin, Notch, and JAK/STAT, the main takeaway is that cancer stem cells can resist therapies through quiescence, drug efflux, and anti-apoptosis[21]. Overall, these mechanisms make CSCs difficult to target. When combined with hormone receptor therapy resistance, these qualities make TNBC an especially difficult disease to treat. Consequently, understanding the cellular biology of CSCs in TNBC, and other cancers, can provide insight into future novel therapies[22,23].
GD2: a cancer stem cell biomarker
Stem cells are essential for their abilities to self-renew and differentiate and are responsible for migrating to biological niches to replace damaged or depleted cell types[24]. In cancer, these same qualities lead to disease progression, metastasis, and patient mortality. It is important, then, to characterize CSC-specific cell surface markers for targeted treatment. In 2003, Al-Hajj et al. identified CD44high/CD24low as breast CSC markers[25]. Shortly after, ALDH1 was identified as another breast CSC marker, although primarily for luminal subtypes[26]. However, these markers are poor targets for therapy because they are also expressed by normal human breast cells[27].
In 2012, Battula et al. identified GD2 as a novel breast cancer stem cell marker in TNBC and that it is co-expressed with CD44high/CD24low cells[28]. GD2, a b-series ganglioside located within lipid rafts on the outer side of the plasma membrane, is expressed during development but is highly restricted to cerebellar tissues and peripheral nerves in mature adults. It can also be found on tumors of neuroectodermal origin such as neuroblastoma and melanoma[29,30]. Battula et al. and Jaggupilli et al. then went on to identify NF-kB signaling, an inflammatory pathway, and metabolic stress, respectively, as primary drivers of GD2 expression[31,32]. Further studies by Nguyen et al. identified GD2 as an activator of the FAK-AKT-mTOR signaling pathway, leading to increased tumor growth and metastasis in in vivo conditions[33]. Furthermore, this study demonstrated that CRISPR knock-out of GD3S, the rate-limiting enzyme for GD2 synthesis, completely ablated GD2 cells. Interestingly, GD3S KO cells resulted in no in vivo tumor growth and metastasis, greatly reduced in vitro migration, invasion, and growth in 3D conditions, but had no effect on in vitro 2D proliferation. Patient studies have shown that high levels of GD2 have been associated with advanced cancer stage, larger tumor size, nodal invasion, and enhanced tumor proliferation and invasiveness[34]. These clinical correlations make GD2 an ideal breast CSC therapeutic target.
Targeting GD2 to treat cancer stem cells
Characterized by its lack of hormone receptor status, patients diagnosed with TNBC are typically unresponsive to target-specific hormone receptor therapies. Treatment options primarily consist of surgery, neoadjuvant therapies, and chemotherapy with standard cytotoxic agents. Despite initial responses, the overall prognosis remains poor[35,36]. GD2 has been identified as a suitable therapeutic biomarker for targeting breast CSCs because of its limited expression in normal, healthy tissues. Because of this, therapies that target GD2 could be a promising avenue for treatment development. Battula et al. identified BMS-345541 as a potential upstream small molecule inhibitor for GD2 by blocking IKK activity, thus suppressing NF-kB signaling[31]. Although NF-kB is ubiquitous in normal tissues and cells, it is typically inactive until cellular stress responses such as inflammation occur. Furthermore, Jaggupilli et al. were able to reduce the GD2+ CSC population by 70%-80% using glutamine uptake inhibitor, V9302[32]. Lastly, Nguyen et al. were able to suppress the activity of GD2+ CSCs by inhibiting downstream activation of FAK and mTOR using PF-573228 and everolimus, respectively[33].
In addition to targeting the upstream and downstream mechanisms of GD2 expression and activity, immune-based therapies that target GD2 expressing cells could also be pursued. Seitz et al. accomplished this by developing CAR T-cells (chimeric antigen receptor T-cells) that are genetically engineered to target GD2 expressing cells[37]. Although their studies demonstrated anti-GD2 CAR T-cells to be effective at reducing metastasis in vivo, graft-versus-host disease (GVHD) is a major cause of clinical morbidity and mortality[38]. To overcome the limitations associated with T-cells, natural killer (NK) cells could be generated instead. Esser et al. demonstrated that engineered GD2-specific NK cells were able to induce ADCC-like (antibody-dependent cellular cytotoxicity) killing effects against neuroectodermal tumors[39]. Furthermore, Ly et al. determined that infusion of anti-GD2 antibody dinutuximab, a chimeric human-mouse monoclonal antibody, could inhibit tumor growth in vivo and extend the survival of mice with TNBC via NK cell-mediated antibody-dependent cellular cytotoxicity[34]. The FDA has already approved GD2-specific therapies for the treatment of neuroblastoma, osteosarcoma, and other GD2+ cancers. Additionally, other anti-GD2 therapies, such as naxitamab and an anti-GD2-GD3 vaccine, have been granted FDA approval[40,41]. Anti-GD2 antibodies, however, do confer toxicity and are associated with adverse side effects including neuropathy, fever, significant pain, and allergies[42]. Neuropathic pain is likely due to GD2 expression in neural cells; however, O-acetyl-GD2 (OAcGD2), a GD2 derivative, is expressed on GD2+ solid tumor cells but not on neural fibers, and specific targeting OAcGD2 may reduce the aforementioned side effects[43]. Overall, these compounds may be beneficial for the treatment of TNBC, in combination with other therapies, by targeting the CSC subpopulation.
SUMMARY
Breast cancer is now the most commonly diagnosed cancer, accounting for 12% of global cases. Although advances in breast cancer treatment have been made, treatment resistance remains a problem for many patients. This is especially true for patients diagnosed with triple-negative breast cancer because of this cancer subtype’s inherent ability to resist hormone receptor targeted therapies. Additionally, treatment with chemo- and radiotherapies is selected for the cancer stem cell subpopulation, a subset of cancer cells that is resistant due to mechanisms involving quiescence, DNA replication and repair, and apoptosis. Furthermore, these CSCs have an increased tumorigenic and metastatic potential.
GD2 has been identified as a breast cancer stem cell marker and is a promising target for breast cancer therapy[44]. Furthermore, dinutuximab is a GD2-specific monoclonal antibody that is FDA approved for neuroblastoma. Because of this, we believe incorporating methods for targeting CSCs, specifically by GD2, into current TNBC research may lead to promising results. Examples of this would be GD2 combination therapies with novel pathway inhibitors, analyzing the effects in the tumor microenvironment, and studying the role of CSCs on the tumor architecture by looking at the extracellular matrix. Any discoveries could potentially lead to rapid clinical turnarounds due to the FDA-approved status of dinutuximab.
DECLARATIONS
Authors’ contributionsContributed to the conception and writing of this manuscript: Nguyen K
Contributed to reviewing the latest literature in the field, writing, and generation of the graphical abstract: McConnell E, Edwards O
Contributed their experience and gave final approval for submission: Collins-Burow BM, Burow ME
Availability of data and materialsNot applicable.
Financial support and sponsorshipWe acknowledge and thank Krewe de Pink for their support and dedication to breast cancer patients in the local New Orleans community and to breast cancer research.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. U.S. Breast cancer statistics. 2022. Available from: https://www.breastcancer.org/ [Last accessed on 7 Jun 2022].
2. Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 2020;21:3233.
3. Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist 2019;2:141-60.
4. Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. The Breast 2015;24:S26-35.
5. Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. J Cancer Res Pract 2015;5:2929-43.
6. Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 2015;21:1688-98.
7. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750-67.
8. Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res 2020;22:61.
10. Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol 2011;9:16-32.
11. Al-Mahmood S, Sapiezynski J, Garbuzenko OB, Minko T. Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res 2018;8:1483-507.
12. Yu KD, Zhu R, Zhan M, et al. Identification of prognosis-relevant subgroups in patients with chemoresistant triple-negative breast cancer. Clin Cancer Res 2013;19:2723-33.
13. McGranahan N, Swanton C. Clonal Heterogeneity and tumor evolution: past, present, and the future. Cell 2017;168:613-28.
16. Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 2008;10:R25.
17. Malladi S, Macalinao DG, Jin X, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 2016;165:45-60.
18. Das S, Mukherjee P, Chatterjee R, Jamal Z, Chatterji U. Enhancing chemosensitivity of breast cancer stem cells by downregulating SOX2 and ABCG2 using wedelolactone-encapsulated nanoparticles. Mol Cancer Ther 2019;18:680-92.
19. Sun M, Yang C, Zheng J, et al. Enhanced efficacy of chemotherapy for breast cancer stem cells by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense. Acta Biomater 2015;28:171-82.
20. Takeda H, Okada M, Suzuki S, et al. Rho-Associated Protein Kinase (ROCK) inhibitors inhibit survivin expression and sensitize pancreatic cancer stem cells to gemcitabine. Anticancer Res 2016;36:6311-8.
21. Li Y, Wang Z, Ajani JA, Song S. Drug resistance and cancer stem cells. Cell Commun Signal 2021;19:19.
22. Park SY, Choi JH, Nam JS. Targeting cancer stem cells in triple-negative breast cancer. Cancers (Basel) 2019;11:965.
23. Castillo V, Valenzuela R, Huidobro C, Contreras HR, Castellon EA. Functional characteristics of cancer stem cells and their role in drug resistance of prostate cancer. Int J Oncol 2014;45:985-94.
24. Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med 2007;58:267-84.
25. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003;100:3983-8.
26. Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1:555-67.
27. Colacino JA, Azizi E, Brooks MD, et al. Heterogeneity of human breast stem and progenitor cells as revealed by transcriptional profiling. Stem Cell Reports 2018;10:1596-609.
28. Battula VL, Shi Y, Evans KW, et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J Clin Invest 2012;122:2066-78.
29. Wierzbicki A, Gil M, Ciesielski M, et al. Immunization with a mimotope of GD2 ganglioside induces CD8+ T cells that recognize cell adhesion molecules on tumor cells. J Immunol 2008;181:6644-53.
30. Bolesta E, Kowalczyk A, Wierzbicki A, et al. DNA vaccine expressing the mimotope of GD2 ganglioside induces protective GD2 cross-reactive antibody responses. Cancer Res 2005;65:3410-8.
31. Battula VL, Nguyen K, Sun J, et al. IKK inhibition by BMS-345541 suppresses breast tumorigenesis and metastases by targeting GD2+ cancer stem cells. Oncotarget 2017;8:36936-49.
32. Jaggupilli A, Ly S, Nguyen K, et al. Metabolic stress induces GD2+ cancer stem cell-like phenotype in triple-negative breast cancer. Br J Cancer 2022;126:615-27.
33. Nguyen K, Yan Y, Yuan B, et al. ST8SIA1 Regulates tumor growth and metastasis in TNBC by activating the FAK-AKT-mTOR signaling pathway. Mol Cancer Ther 2018;17:2689-701.
34. Ly S, Anand V, El-Dana F, et al. Anti-GD2 antibody dinutuximab inhibits triple-negative breast tumor growth by targeting GD2+ breast cancer stem-like cells. J Immunother Cancer 2021;9:e001197.
35. De Laurentiis M, Cianniello D, Caputo R, et al. Treatment of triple negative breast cancer (TNBC): current options and future perspectives. Cancer Treatment Reviews 2010;36:S80-6.
36. Isakoff SJ. Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J 2010;16:53-61.
37. Seitz CM, Schroeder S, Knopf P, et al. GD2-targeted chimeric antigen receptor T cells prevent metastasis formation by elimination of breast cancer stem-like cells. Oncoimmunology 2020;9:1683345.
38. Ghosh A, Smith M, James SE, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med 2017;23:242-9.
39. Esser R, Müller T, Stefes D, et al. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med 2012;16:569-81.
41. Cheung IY, Cheung NV, Modak S, et al. Survival impact of anti-GD2 antibody response in a phase II ganglioside vaccine trial among patients with high-risk neuroblastoma with prior disease progression. J Clin Oncol 2021;39:215-26.
42. Sorkin LS, Otto M, Baldwin WM 3rd, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain 2010;149:135-42.
43. Cavdarli S, Dewald JH, Yamakawa N, et al. Identification of 9-O-acetyl-N-acetylneuraminic acid (Neu5,9Ac2) as main O-acetylated sialic acid species of GD2 in breast cancer cells. Glycoconj J 2019;36:79-90.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Nguyen K, McConnell E, Edwards O, Collins-Burow BM, Burow ME. GD2+ cancer stem cells in triple-negative breast cancer: mechanisms of resistance to breast cancer therapies. Cancer Drug Resist 2022;5:721-6. http://dx.doi.org/10.20517/cdr.2022.30
AMA Style
Nguyen K, McConnell E, Edwards O, Collins-Burow BM, Burow ME. GD2+ cancer stem cells in triple-negative breast cancer: mechanisms of resistance to breast cancer therapies. Cancer Drug Resistance. 2022; 5(3): 721-6. http://dx.doi.org/10.20517/cdr.2022.30
Chicago/Turabian Style
Nguyen, Khoa, Emily McConnell, Orielle Edwards, Bridgette M. Collins-Burow, Matthew E. Burow. 2022. "GD2+ cancer stem cells in triple-negative breast cancer: mechanisms of resistance to breast cancer therapies" Cancer Drug Resistance. 5, no.3: 721-6. http://dx.doi.org/10.20517/cdr.2022.30
ACS Style
Nguyen, K.; McConnell E.; Edwards O.; Collins-Burow BM.; Burow ME. GD2+ cancer stem cells in triple-negative breast cancer: mechanisms of resistance to breast cancer therapies. Cancer Drug Resist. 2022, 5, 721-6. http://dx.doi.org/10.20517/cdr.2022.30
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 6 clicks
Cite This Article 6 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.