Liquid biopsies in primary and secondary bone cancers
Abstract
Liquid biopsies are a powerful tool to non-invasively analyze tumor phenotype and progression as well as drug resistance. In the bone oncology field, liquid biopsies would be particularly important to develop, since standard biopsies can be very painful, dangerous (e.g., when found in proximity to the spinal cord), and hard to collect. In this review, we explore the recent advances in liquid biopsies in both primary (osteosarcoma and Ewing sarcoma) and secondary bone cancers (breast, prostate, and lung cancer-induced bone metastases), presenting their current role and highlighting their unexpressed potential, as well as the barriers limiting their possible adoption, including costs, scalability, reproducibility, and isolation methods. We discuss the use of circulating tumor cells, cell-free circulating tumor DNA, and extracellular vesicles for the purpose of improving diagnosis, prognosis, evaluation of therapy resistance, and driving therapy decisions in both primary and secondary bone malignancies.
Keywords
INTRODUCTION
Normal bone physiology: cellular players and the “virtuous cycle”
Bone is a connective tissue composed of a mineralized matrix and a cellular component. The former is made up of an inorganic part (mainly calcium phosphate) and an organic one, composed of type I collagen fibers as well as a plethora of proteins such as osteonectin, osteopontin, osteocalcin and proteoglycans. This bone matrix is also a storage for several growth factors belonging to the transforming growth factor (TGF)/insulin-like growth factor (IGF) superfamilies[1,2]. The two matrix components make bone a composite material with outstanding mechanical properties, but this comes with a caveat: bone needs to be modeled and remodeled during growth and adult life, respectively, otherwise its mechanical properties degrade over time, as is the case for elderly individuals of both genders and for some bone genetic diseases[3,4]. Proper bone modeling/remodeling is guaranteed by a finely tuned balance among the three main bone cells: osteoblasts, the bone-forming cells; osteoclasts, the bone-resorbing cells; and osteocytes, key controllers of bone matrix homeostasis[5,6]. During bone modeling, all bone sections change shape following both mechanical cues and microenvironmental factors and expand to accommodate the growing organism in physiological conditions. In adults, bone switches to remodeling, where osteoblasts and osteoclasts act on the same surfaces and constantly resorb and depose new bone, resulting in a net bone mass balance, following physiological mechanical stimuli as well as the organismal needs for calcium and phosphate[7]. Although this might appear to be a waste of energy, it is crucial to maintain proper mechanical features and guarantee the repair of microfractures that are detected by osteocytes, which subsequently drive the remodeling of the damaged surfaces. Osteocytes are also able to sense mechanical loading, which acts as an anabolic stimulus that promotes osteoblast activity[8,9]. Since the process of bone resorption-deposition in homeostatic conditions keeps bone at its best, and it is cyclic, bone researchers often use the term “virtuous cycle” to describe it.
Primary bone tumors
Osteosarcoma is the most common primary bone tumor. It has two separate incidence peaks, the highest one between 10 and 20 years of age and a secondary one in people aged ≥ 65 years [10]. Although the overall incidence of this cancer is low (3.1 cases per million per year in the US)[11], it represents 2% of all cancers in 0-14-year-old and 3% in 14-19-year-old[12], and its consequences can be devastating, ranging from limb amputation (20% of operable osteosarcomas) to death because of lung metastases[11]. Osteosarcoma derives from a malignant transformation of the mesenchymal cells that can present different cellular features, but they all have in common the deposition of osteoid-like matrix and mineralized or demineralized lesions that are clearly visible upon X-ray examination[13]. When the gold standard treatment, i.e., neo-adjuvant chemotherapy-surgery-adjuvant chemotherapy, is not possible, the only available treatment course is chemotherapy alone, since radiotherapy does not provide significant benefits and increases the risk of infection[14]. However, despite advances in the field, chemoresistance is a serious problem in this type of neoplasia, and the survival benefits of chemotherapy are limited due to the onset of drug resistance[14-16], which eventually leads to lung metastasis development, inevitably ending with the death of the patient. Chemotherapy is therefore a limited tool in osteosarcoma management, but having a reliable way to monitor the onset of drug resistance would make it much more effective.
There is still no consensus on the cell from which Ewing sarcoma originates, but what is certain is that it most often localizes in bone (80%-85%) or the soft tissue surrounding it (15%-20%)[17]. Ewing sarcoma also has quite a high incidence of 2.93 per million in newborns to adolescents[18]. Moreover, 85% of Ewing sarcomas harbor a specific chromosomal translocation [t(11:22)(q24:q12)] leading to the fusion of the N-terminal portion of Ewing sarcoma gene (EWS) with the C-terminal portion of Friend leukemia integration 1 transcription factor (FLI1), which encodes for a chimeric protein that behaves as a transcription factor and modulates a plethora of cellular processes, eventually leading to malignant transformation[17]. While most other bone cancers have specific morphological features that make tracking their lineage relatively easy, Ewing sarcoma cells are round, small basophils and defined as “uniformly undifferentiated”[17]. Despite advances in the field of Ewing sarcoma treatment, 70% of metastatic patients, in whom lungs are most frequently affected as a secondary site, will eventually perish[19]. Treatment of this tumor is multimodal and comprises surgery, radiotherapy, and chemotherapy[20]. However, drug resistance is a very concrete issue, which sadly tends to increase after more aggressive rounds of chemotherapy that, in fact, do not seem to provide significant survival benefits and is especially marked in relapsing Ewing sarcoma[21,22]. Hence, monitoring the onset of drug resistance with a non-invasive technique such as liquid biopsy could be a valuable tool in the fight against this family of tumors as well.
Bone metastases and the “vicious cycle”
The bone milieu is a particularly attractive microenvironment for metastasis development by many primary tumors, such as breast, prostate, lung, and kidney cancers[23-25]. There could be several reasons for this “preference”, such as the richness in growth factors of the bone matrix[26], the presence of higher levels of calcium, which acts as a growth-promoting factor[27,28] especially following bone resorption, and the particular structure of bone/bone marrow blood vessels, which are fenestrated, hence quite permissive for tumor cells extravasation. In fact, metastasis is a stepwise process, in which first cancer cells have to invade locally in the primary site, after gaining the expression of specific sets of molecules such as matrix metalloproteinases[29,30], migrate into the bloodstream or lymphatic system, escape immune killing while circulating, extravasate in the secondary site, engraft, and finally survive in the secondary organ. The final parts of the process, spanning from the extravasation to the survival in the new microenvironment, are often termed “homing” and require cancer cells to “trick” the resident cells into believing that they actually belong there, expressing bone-specific factors (osteomimicry)[31] or hematopoietic stem cell-specific factors (HSC-mimicry)[32].
Depending on the type of cancer, osteoblasts, osteoclasts, and osteocytes are affected in specific ways. In the case of breast cancer, metastatic dissemination usually causes the local degradation of the bone matrix, which is evident on X-ray analysis as void areas where bone should be. These types of metastases are termed “osteolytic”, and while both osteoblasts and osteoclasts are involved in their establishment, the main culprit for their radiographic appearance and fueling are osteoclasts, which become overactivated following their interaction with cancer cells[33,34]. On the other hand, prostate cancer preferentially causes a net activation of osteoblasts in the bone metastatic microenvironment, leading to abnormally high bone deposition and the onset of radio-dense spots identifiable by X-ray examination. These metastases are termed “osteosclerotic” or “osteoblastic”[24]. Both cancers can present osteolytic and osteosclerotic features in the same anatomical site, and in this case, bone metastases are defined as “mixed”[23]. The process leading to osteolytic or osteosclerotic lesions relies on a pathological cross-communication between cancer cells and bone cells, where the former tilt the balance towards bone resorption or bone deposition, hijacking the virtuous cycle of cross-regulation between osteoblasts and osteoclasts into an osteolytic or osteosclerotic “vicious cycle”.
In the former, cancer cells that reached the bone/bone marrow microenvironment secrete factors that induce a net increase in osteoclast differentiation and activity. These factors can act directly [e.g., tumor necrosis factor-α, interleukin (IL)-6, and IL-1β], and/or indirectly [like parathyroid hormone-related protein (PTHrP)], through the promotion of osteoblastic expression of pro-osteoclastogenic factors such as receptor activator of nuclear factor κB (RANKL) and macrophage colony-stimulating factor (M-CSF)[26,33-35]. The increase in osteoclast activity and differentiation leads to the degradation of bone matrix and the release of IGF-1, TGF-β, platelet-derived growth factor (PDGF), and bone morphogenetic proteins (BMPs) from it, which in turn stimulate tumor growth, thus creating a feed-forward loop, with bone cells, i.e., the osteolytic vicious cycle. In the osteosclerotic vicious cycle, cancer cells secrete a different set of proteins, including IGF-1, wingless-related integration site-1 (WNT-1) and WNT-3A, BMPs, and endothelin (ET)-1. These promote osteoblast differentiation and activity, leading to deposition of primary bone and production of growth factors[24,36,37]. Tumor growth is therefore fomented by osteoblasts, thus closing the osteosclerotic vicious cycle. Osteoblast differentiation also stimulates osteoclast differentiation, which further exacerbates the release of matrix-bound growth factors, with dangerous and painful consequences[23,37].
LIQUID BIOPSY IN BONE ONCOLOGY
Liquid biopsies: what, how and why
Liquid biopsies are obtained in body fluids that are in direct contact with cancerous tissue and can provide important insights into the tumor without having to remove a part of it directly[38,39]. This is especially important for those tumors that are not easily accessible and for which even drawing a simple biopsy core may be dangerous and/or extremely painful. Furthermore, classical biopsies may be taken from a relatively small area of the tumor and may not be representative of it as a whole[40]. Although depending on the neoplasia might be useful to use urine, cerebrospinal fluid, and even saliva as a liquid biopsy, the body fluid that is most useful for this application is blood[39]. An additional advantage of using liquid biopsies from blood is that they can be multiplexed and implemented with classical as well as next-generation cancer profiling analyses, including circulating bone turnover biomarkers such as carboxy-terminal telopeptide cross-linked type 1 collagen, pro-peptide of type 1 collagen, bone sialoprotein, tartrate-resistant acid phosphatase 5B (TRAcP5B), and osteoprotegerin[41,42]; metabolites such as pyridinoline and deoxypyridinoline[43]; and protein markers correlating with poor outcome in bone cancers, including vascular endothelial growth factor (VEGF)[44], metallothionein[45], IL-4, and IL-8[46,47]. There are three main types of actionable biological materials that can be obtained from a blood liquid biopsy: (i) circulating tumor cells (CTCs); (ii) cell-free circulating tumor DNA (ctDNA); and (iii) extracellular vesicles (EVs, also referred to as exosomes)[48].
CTCs
As the name suggests, CTCs are tumor cells that extravasate into the blood flow. They were first identified by Ashworth more than 150 years ago and are now commonly used in clinical practice[49]. Although isolating CTCs has historically been a challenge because of their low number in the general circulation, newly developed microfluidic platforms (e.g., the FDA-approved CellSearch platform) are making this task easier, and next-generation sequencing approaches made it possible to deeply phenotype every single circulating tumor cell isolated[50]. These important advances mean that a simple liquid biopsy can provide information about the genetic mosaicism of the primary tumor, their mutational landscape, epigenetics, and even their gene and protein expression.
Circulating tumor (ct)DNA
Cell-free ctDNA arises from apoptotic and necrotic primary tumor cells, which release their cellular content into the general circulation, as demonstrated by the fact that most of the ctDNA detected is 180-200 bp in length, which is consistent with what is observed in apoptotic cells[48]. Circulating tumor DNA can provide important information about tumor mutations and copy number variation and suggests whether to proceed with a specific-target therapy or not. For example, the V600E mutation in v-raf murine sarcoma viral oncogene homolog B1 (BRAF) is found in several tumors, including metastatic colorectal cancer[51], melanoma[52,53], papillary thyroid cancer[54], etc. The V600E variant of BRAF is targetable with drugs such as vemurafenib, dabrafenib, and trametinib, and its presence can drive the choice of therapy[55].
Extracellular vesicles
The third class of biological material that is usable as liquid biopsy includes extracellular vesicles. These are lipid bilayer particles ranging from 30 to 1000 nm in diameter, which, according to their size, can be classified into three main types: apoptotic bodies, large extracellular vesicles (also known as microvesicles), and small EVs (also known as exosomes). These also differ in biogenesis and biological function[56,57]. Importantly, EVs secreted by cells often mimic the molecular composition of the cell of origin, and they contain DNA, miRNAs, mRNAs, proteins, and other biological molecules that are interesting for theranostic and prognostic purposes[56,58]. It has been thoroughly demonstrated that EVs have an important role in cancer[59,60], including bone metastases[60-63], osteosarcoma[64-66], and Ewing sarcoma[67,68], and cancer cells secrete more EVs than their normal counterparts[69]. Moreover, hypoxia, higher intracellular calcium or lower pH, oxidative stress, ionizing radiation, and ultrasounds have all been shown to increase EV production in both normal and cancer cells[70].
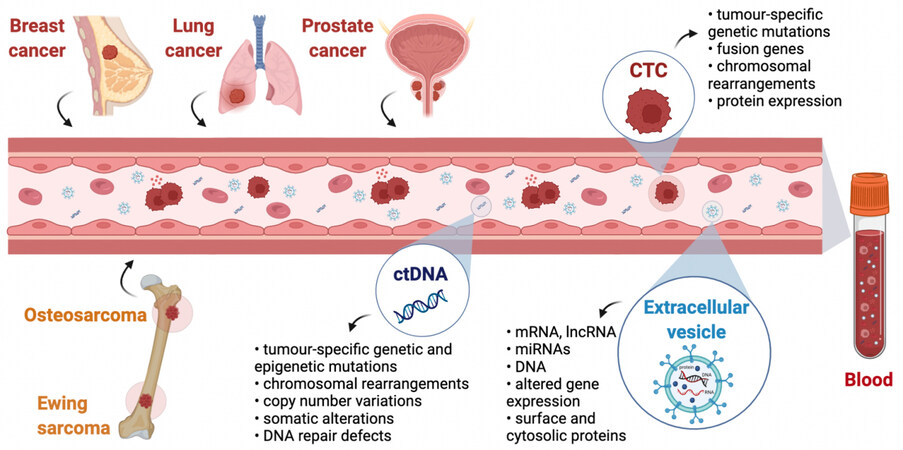
EVs are especially interesting for studying RNAs because these molecules are prone to be degraded in the circulation; however, when they are encapsulated into EVs, they become more stable and can be analyzed to gain insights into the transcriptional profile of the cells of origin, as well as regarding miRNAs and long non-coding RNAs[71]. A summary of the primary and secondary tumors and the potential information that can be gained by liquid biopsies is schematized in Figure 1.
Figure 1. Liquid biopsies in primary and secondary bone cancers. Cell-free ctDNA, CTCs, and EVs are released into the general circulation by both primary (osteosarcoma and Ewing sarcoma) and secondary (breast, lung, and prostate) cancers and can be collected with a simple blood draw. Studying these three cancer-derived components by molecular or cellular analysis is termed liquid biopsy. Much information about the tumor can be achieved by analyzing CTCs, ctDNA, and EVs, making liquid biopsies a minimally invasive alternative to classical biopsies. Created with BioRender.com. ctDNA: Circulating tumor DNA; CTCs: circulating tumor cells; EVs: extracellular vesicles.
Adopting different approaches to acquire all this biological information from a patient’s blood could be possible to perform accurate diagnosis, estimate prognosis, and even monitor the onset of drug-resistant clones to make informed therapeutic choices and change therapy during treatment[72].
Despite the advances in the field at the preclinical level, clinical adoption of bone liquid biopsies remains poor due to costs, scalability, reproducibility, and isolation methods. In the following sections, we focus on the recent developments, as well as preclinical and clinical applications of liquid biopsies in bone oncology, focusing on both secondary [Table 1] and primary [Table 2] tumors.
Selected studies about liquid biopsy applications in bone metastatic tumors
| Tumor type | Source material | Target | Utility/use | References |
| Breast cancer | ctDNA | Tumor chromosomal rearrangements, ESR1 mutations | Early diagnosis of BM | [74,92] |
| ctDNA | TP53, PIK3CA, ESR1 mutations | Prognosis and treatment efficacy | [89,93] | |
| ctDNA | PIK3CA mutations | Diagnosis of BM | [90] | |
| ctDNA | Somatic genomic alterations (PIK3CA and TP53) | Prognosis and treatment efficacy | [91] | |
| CTCs | Baseline CTC/mL of blood (≥ 5 CTC/7.5 mL) | Diagnosis and prognosis of BM | [80,81,89] | |
| EVs | Upregulation of SPP1, HSP90AA1, IL3, VEGFA, PTK2 and YWHAZ genes | Early detection of BM | [94] | |
| Lung cancer (NSCLC) | ctDNA | KRAS, EGFR, BRAF mutations | Early diagnosis of BM | [77] |
| ctDNA | KRAS and EGFR mutations | Diagnosis and prognosis of BM | [78] | |
| CTCs | CTCs/mL of blood (≥ 5 CTC/7.5 mL) | Diagnosis and prognosis of BM | [84,85] | |
| EVs/cmiRNAs | hsa-miR-574-5p, hsa-miR-328-3p, hsa-miR-423-3p | Early detection and monitoring of BM | [95] | |
| Prostate cancer | ctDNA | TP53 mutations and DNA repair defects | Diagnosis of BM | [79] |
CTCs | CTCs/mL of blood (≥ 5 CTC/7.5 mL) | Diagnosis and prognosis of BM | [86] | |
| EVs/cmiRNAs | miR-181a-5p | Diagnosis of BM | [96] |
Selected liquid biopsy applications in primary bone tumors
| Tumor type | Source material | Target | Utility/use | References |
| Osteosarcoma | ctDNA | Somatic mutations | Diagnosis and prognosis | [100] |
| ctDNA | Chromosome arm 8q copy number gains | Genotyping and diagnosis | [101] | |
| CTCs | Baseline CTC/mL of blood (≥ 5 CTC/7.5 mL) | Diagnosis, prognosis, response to therapy | [104,105] | |
| CTCs | CTC count variations after therapy | Prognosis and treatment efficacy | [106,107] | |
| EVs | Transcriptomic alterations | Diagnosis of BM | [111] | |
| EVs/cmiRNAs | miR-148a, -574-3p, -214, -335-5p, -491, -221, -191, -421, -124, -101 and -195 | Diagnosis and prognosis | [112] | |
| Ewing sarcoma | ctDNA | STAG2 and TP53 mutations, EWSR1-FLI1 and EWSR1-ERG fusion genes | Diagnosis, prognosis and response to therapy | [102,103] |
| CTCs | CTCs count, CD99 expression and chromosomal translocations (EWSR1- FL11 fusion gene) | Diagnosis and prognosis of metastasis | [108] | |
| EVs/cmiRNAs | miR-125b | Diagnosis, prognosis and response to therapy | [121] | |
| EVs | Proteomic content (CD99, HINT1 and NGFR) | Diagnosis and prognosis | [122] |
Liquid biopsy in bone metastasis
All the key components in liquid biopsies, i.e., ctDNA, CTCs, and EVs, have been exploited to monitor bone metastases secondary to breast, prostate, and lung cancers at the preclinical level.
ctDNA for bone metastasis detection
Circulating tumor DNA analysis may provide a complete genetic profile of the mutational landscape of metastatic disease, and is also correlated with patients’ relapse or changes in response to surgical or pharmacological treatment[73]. In this regard, a retrospective study of patients with primary breast cancer showed that the detection of metastatic disease, also spread in the bone, was possible by serial measurements of selected tumor-specific chromosomal rearrangements in ctDNA using droplet-based digital PCR technologies from plasma samples, with an average of almost one year before clinical recurrence detection during the follow-up of the disease, and the ctDNA amount was directly proportional to poor survival[74]. This highlights the possibility of using ctDNA detection as a diagnostic tool for earlier prediction of metastasis. Liquid biopsies also hold the potential to detect minimal residual disease (MRD), thus providing indications for therapy and prognosis. Detection and analysis of plasma tumor-associated ctDNA were found to be a good indicator of MRD identification and monitoring in breast cancer patients with a high risk of recurrence[75,76]. In particular, the detection of ctDNA at baseline was associated with a higher incidence of bone metastasis and subsequent poor prognosis in newly diagnosed patients with advanced non-small cell lung cancer (NSCLC)[77]. Consistently, quantification and analysis of ctDNA in late-stage NSCLC patients revealed that higher ctDNA levels were detected in the group of patients with bone metastasis[78]. It should be noted that using liquid biopsies as a means of detecting MRD is still a developing field, and the risk of false positives and false negatives is a concrete one that needs to be addressed in larger-scale longitudinal studies. Regarding the use of ctDNA, to the best of our knowledge, there are no studies focusing specifically on prostate cancer bone metastases. However, Vandekerkhove et al. showed that prostate cancer patients with visceral metastases had higher levels of ctDNA than bone metastatic patients[79].
CTCs for bone metastasis detection
As for CTCs, they have also been proven useful for diagnosis, prognosis, and monitoring treatment efficacy in metastatic breast cancer. Indeed, CTC quantification and characterization in patients with metastatic breast cancer have been associated with the presence of bone and liver metastases[80].
Remarkably, higher CTC counts correlated with multiple metastatic sites, while a lower CTC count was found in bone-only metastatic breast cancer patients, who also presented with a better prognosis[81,82], indicating that patients with less advanced disease had fewer CTCs. Moreover, patients with only one or two bone metastases had sharply fewer CTCs compared to patients with more bone metastases[82]. CTCs seem to have a subpopulation of metastasis-initiating cells that express epithelial cellular adhesion molecule, CD44, CD47, and c-MET. Once these cells were sorted and transplanted from a patient to immunocompromised mice, they induced bone, lung, and liver metastases[83].
CTC detection and quantification have shown prognostic potential in lung cancer patients, especially in advanced NSCLC, the most common histological subtype, highly metastasizing to bone. In the last decade, some studies showed that a high number of CTCs is a predictive and prognostic indicator of bone metastasis[84,85] in advanced lung cancer patients. The prognostic utility of CTCs in monitoring prostate cancer bone metastases was validated by multiple prospective studies performed on peripheral blood of metastatic castration-resistant prostate cancer patients after treatment. It was found that CTC counts ≥ 5 per 7.5 mL of blood are associated with lower overall survival and are predictive of bone metastases[86-88].
Some studies suggested a liquid biopsy approach involving simultaneous detection and quantification of both CTCs and ctDNA. A valid example to report is the COMET (NCT01745757) prospective study, conducted on peripheral blood samples collected before and after chemotherapy from a homogeneous group of HER2-negative breast cancer patients. In patients with bone, liver, and brain metastasis, CTCs were greater in number and ctDNA analyses revealed at least one mutation in tumor protein 53, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, and estrogen receptor 1 (ESR1) genes compared to non-metastatic patients[89]. These tumor-specific mutations in ctDNA analysis from plasma of patients with visceral and non-visceral metastasis, including bone, were confirmed in other studies[90-93], making this CTC-ctDNA signature potentially useful for diagnosis and prognosis of metastatic breast cancer.
Extracellular vesicles cargo and miRNAs for bone metastasis detection
As for EVs, starting from a meta-analysis conducted on publicly available microarray datasets derived from multiple-cancer type patients with and without bone metastasis, Bhadresha and colleagues identified 15 genes that were consistently upregulated in bone metastatic patients, and then validated their expression in patient serum-derived EVs. Among these, five were confirmed upregulated by qPCR in EVs isolated from the serum of breast and lung cancer patients harboring bone metastasis, namely, heat shock protein 90 alpha family class A member 1 (HSP90AA1), secreted phosphoprotein 1 (also referred to as osteopontin), IL-3, VEGFA, and protein tyrosine kinase 2. The authors concluded that this EV-derived mRNA gene signature could be a useful predictive tool for the early detection of bone metastases in breast and lung cancers[94]. Few studies focused their attention on EV-derived miRNAs released from lung cancer cells. A retrospective study was conducted on plasma-derived EV miRNAs from NSCLC patients, focusing on their potential as biomarkers of early detection and monitoring of bone metastasis. In particular, hsa-miR-574-5p, hsa-miR-328-3p, and hsa-miR-423-3p have been suggested as potential biomarkers for bone metastasis[95]. As for prostate cancer, EV-derived miR-181a-5p- was recently found upregulated in bone-metastatic prostate patients by Wang et al. and proposed as a biomarker for its diagnosis; similar findings were shown by Bryant et al. for miR-141 and miR-375[96,97]. Additionally, prostate microparticles, a type of prostate-specific EVs, were found to be more numerous in metastatic prostate cancer compared to non-metastatic cancer and showed better predictive ability than CTCs detected with the FDA-approved CellSearch system[98]. It is worth mentioning that a platform based on urine exosomal gene expression, termed ExoDX, was recently developed and tested in a utility trial on more than 500 patients. ExoDX-based scoring could predict high-grade prostate cancer in patients with uncertain prostate-specific antigen scores better than the gold standard (ROC AUC 0.7 vs. 0.62) and, conversely, it managed to predict which patients only had benign prostate hyperplasia, thus avoiding unnecessary biopsies[99].
Liquid biopsy in primary bone malignancies
ctDNA detection
Few studies have been performed on plasma-derived ctDNAs analysis from osteosarcoma patients[100,101]. One study focused on somatic mutations associated with tumor burden and disease outcome. Here, the researchers, using the targeted next-generation sequencing (NGS) approach, identified tumor-specific somatic alterations by comparing tumor and germline DNA extracted from peripheral mononuclear blood cells with tissue biopsies and demonstrated that patient-specific somatic alterations can be detected in ctDNA collected from the plasma samples at various stages of treatment, which allows the monitoring of disease burden[100]. In another study, Shulman and colleagues showed that ctDNA levels detected by NGS hybrid capture assay in peripheral blood samples of patients with newly diagnosed localized osteosarcoma and Ewing sarcoma may be associated with tumor burden, relapse, and negative disease outcomes. Interestingly, ctDNA analysis led to the identification of novel genomic features of osteosarcoma, including chromosome arm 8q copy number gains[101]. Specific and well-characterized genetic mutations, such as stromal antigen 2 (STAG2) and TP53 loss-of-function mutations, translocation events, and fusion genes [Most commonly, EWSR1-FLI1 and EWSR1-erythroblast transformation specific-related gene (ERG)], have been found expressed in Ewing sarcoma patients, leading to the opportunity to monitor this bone malignancy through ctDNA[102]. The above-described retrospective study conducted by Shulman and colleagues showed an association between ctDNA detection in plasma samples and a poor clinical outcome in patients with newly diagnosed Ewing sarcoma and identified from ctDNA analysis additional genomic information, such as EWSR1 fusion and STAG2 loss-of-function mutations[101]. Another promising application for liquid biopsy in Ewing sarcoma was provided by Hayashi et al., who found that tumor burden and response to therapy were related to increased levels of circulating EWSR1-FLI1 fusion gene in plasma of patients. Moreover, they observed that EWS-FLI1 levels in the circulation decreased after chemotherapy or surgery and then started to rise again during tumor recurrence[103].
CTCs analysis
CTCs have been proposed as potential predictive and prognostic markers for osteosarcoma metastasis[104]. A prospective study undertaken by Li et al. revealed a higher number of CTCs detected at baseline in peripheral blood of metastatic osteosarcoma patients compared to ones with localized disease. Moreover, they observed that CTC count was inversely correlated with the patient response after neoadjuvant chemotherapy[105]. Consistently, other preclinical studies have shown that CTC count variations after therapy or surgical resection can reflect the tumor’s sensitivity to the treatment and may be a good indicator of metastasis[106,107], highlighting the clinical interest in dynamic monitoring of CTC changes for understanding treatment efficacy and detecting disease recurrence or metastasis in time. In particular, the presence of an increased percentage of CTCs with mesenchymal phenotype (identified by the epithelial-to-mesenchymal transition markers) in peripheral blood of a small group of osteosarcoma patients after chemotherapy treatment was associated with reduced disease-free survival, leading to the possibility to predict disease relapse and lung metastasis occurrence[106,107].
A few studies have reported the use of tumor-specific makers for CTC isolation and characterization in Ewing sarcoma patients, including CD99 expression and presence of chromosomal translocations, such as amplification of EWSR1-FLI1 transcript fusion gene, by using different methods[108,109]. Others have suggested that detection of CTCs at diagnosis in Ewing sarcoma patients may be associated with worse clinical outcomes and increased risk of recurrent disease or development of overt metastasis[109,110].
Extracellular vesicles cargo and miRNAs
EVs have recently been studied as diagnostic or prognostic serum biomarkers via a liquid biopsy approach in osteosarcoma. Circulating EVs RNA profiling of metastatic vs. primary osteosarcoma samples allowed the detection of multiple transcriptomic alterations in the former, providing a new clinically relevant approach to track metastatic osteosarcoma[111].
Several miRNAs, which are known to at least partially circulate inside EVs, with oncogenic or antitumor-suppressor roles in osteosarcoma, have been detected in the peripheral blood of patients. Some of them are emerging as important diagnostic and prognostic biomarkers, such as miR-148a[112], miR-574-3p, miR-214 and miR-335-5p[113], miR-491[114], miR-221[115], miR-191[116], and miR-421[117]. Conversely, miR-124[118], miR-101[119], and miR-195[120] were shown to be downregulated in the serum of osteosarcoma patients, compared to healthy individuals. A potential application for these findings could be to establish a predictive strategy for osteosarcoma prognosis using a combination of these miRNAs. Circulating miRNAs have also recently become the subject of study in Ewing sarcoma. As an example, a widely studied circulating miRNA related to Ewing sarcoma progression is miR-125b, which was found decreased in patients serum after surgical resection when compared to healthy controls[121]. In the same study, its downregulation in the group of patients analyzed was also correlated with poor response to chemotherapy[121]. Recently, the research focus is shifting towards Ewing sarcoma-derived EV cargo as a prognostic biomarker source, particularly to their protein content. Samuel et al. identified and used CD99, histidine triad nucleotide-binding protein 1 (HINT1), and nerve growth factor receptor (NGFR) membrane proteins as potential biomarkers of Ewing sarcoma-derived small EVs. They developed an approach of immuno-enrichment of Ewing sarcoma-associated small EVs based on these EV surface proteins, for the subsequent detection of EWS-FLI1 and EWS-ERG fusion transcripts in EVs isolated from plasma of both localized and metastatic patients[122].
CLINICAL IMPLICATION OF LIQUID BIOPSY IN MONITORING DRUG RESISTANCE
Liquid biopsies in chemoresistance of primary and secondary bone cancers: an overview
A growing body of evidence suggests that the tumor secretome, including circulating-free DNA fragments from drug-resistant cells containing tumor-specific genetic and epigenetic mutations, is highly abundant in plasma; thus, the role of blood-based liquid biopsy is fundamental for this field of research[123]. Although several studies have been conducted on plasma samples of relatively small cohorts of patients, trying to identify resistance mutations that occur during treatment, the data obtained thus far are clinically informative about therapy response, but still not completely validated in clinical practice. According to the different types of tumors, quantification and analysis of ctDNA were found to be usable as a viable tool for this purpose[124].
A good example suggesting that ctDNA is a valuable strategy to monitor treatment efficacy was provided by Schiavon et al., who showed that ESR1 mutations found in ctDNA from plasma of metastatic breast cancer patients previously treated with aromatase inhibitors are associated with resistance to endocrine therapy and shorter progression-free survival[125]. Additionally, liquid biopsy has also proved to be useful for the identification of biomarkers associated with cyclin-dependent kinase inhibitors (CDKi) resistance and for predicting the subsequent development of metastatic disease, in hormone receptor-positive/human epidermal growth factor receptor 2 negative (HR+/HER2-) advanced breast cancer patients. Patients treated with CDKi in combination with endocrine therapy presented with specific therapy-induced mutations in the ctDNA analyzed, including retinoblastoma, ESR1, fibroblast growth factor receptor 1, or phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha alterations[126-128], which could be useful for predicting disease outcomes and drive therapeutic decisions. It is also important to mention the detection and quantification of CTC acquired resistance, which may also act as a prognostic marker able to predict treatment outcomes[129]. Similarly, in castration-resistant prostate cancer patients treated with docetaxel, CTC count in blood was found to be an effective indicator of treatment sensitivity and patient survival[130].
Due to its high genetic instability, osteosarcoma treatment is often hampered by the acquisition of chemoresistance following therapy-induced selective pressure. Some studies observed that low serum levels of miR-375 in osteosarcoma patients were related to poor tumor response to preoperative chemotherapy[131]. Recently, other tumor-associated miRNAs have been linked with osteosarcoma chemoresistance, such as miR-491, which was found to be decreased in serum from osteosarcoma patients compared with healthy control subjects, and this decreased serum miR-491 level is correlated with increased metastasis, poor chemoresponse, and lower survival rate[114]. In contrast, the serum levels of miR-21 were found to be significantly higher in patients with osteosarcoma than in control subjects and correlated with advanced Enneking stage and chemotherapeutic resistance[132].
As for the development of chemoresistance in Ewing sarcoma, a recent study showed that circulating miR-125b levels were decreased in patient serum, and this was associated with poor response to chemotherapy[121].
Implication of extracellular vesicles in chemoresistance
Extracellular vesicles are emerging as key players in the transfer of drug resistance[133]; hence, they could be useful for monitoring the onset of this phenomenon during treatment.
Among the most studied constituents of tumor-derived EVs cargo, miRNAs have been identified as potential biomarkers for monitoring chemoresistance. EVs from doxorubicin-resistant osteosarcoma MG63 cells express high levels of the membrane transporter pump P-glycoprotein, transferring resistance to doxorubicin treatment to sensitive cancer cells horizontally[134]. In another report, miR-25-3p upregulation in the blood of osteosarcoma patients correlated with increased tumor growth and drug resistance[135].
Wei and colleagues demonstrated the role of EV-derived miR-222-3p detected in the serum of NSCLC patients for predicting gemcitabine sensitivity and identifying patients with the aggressive advanced and resistant disease[136]. In addition, higher levels of EV-derived miR-425-3p were found in the serum of NSCLC platinum-resistant patients compared with platinum-sensitive patients[137].
In breast cancer, it was found that EV-shuttled miR-222 released by doxorubicin-resistant breast cancer cells was locally transferred to M2 macrophages, thus activating their polarization. In these cells, overexpression of miR-222 suppressed phosphatase and tensin homolog gene, resulting in phosphorylation of Akt and activation of Akt signaling, which in turn supports cancer cells proliferation, migration, and invasion in a positive feedback loop. Accordingly, increased levels of miR-222 were found in EVs from plasma of patients presenting with chemoresistant breast cancer[138]. In another study, it has been demonstrated that the human osteotropic breast cancer cell line MDA-MB-231 treated with paclitaxel was able to release EVs enriched in the cell survival protein Survivin[139].
Interestingly, EVs can also act directly against anti-neoplastic agents. In fact, a recent study showed that HER2-positive breast cancer-derived EVs interfere with the activity of trastuzumab, acting as decoy receptors for it[140]. In fact, EVs secreted by those cancer cells were found to have HER2 on their surface, and trastuzumab administered systemically binds it, hence making the amount of antibody available to bind cells lower. Furthermore, higher levels of glutathione S-transferase P1 (GSTP1) mRNA were found by Yang et al. in EVs from the serum of non-responding breast cancer patients treated with neoadjuvant chemotherapy compared to the responders. Intriguingly, they also demonstrated that GSTP1-containing EVs transferred drug resistance horizontally, and hence proposed their use as negative predictive factors of chemoresistance and clinical outcomes in breast cancer patients treated with anthracycline/taxane-based therapy[141]. Similar results were observed for EV-bound transient receptor potential channel 5 mRNA found in peripheral blood of metastatic breast cancer patients, which could be a potential predictive marker of chemoresistance[142].
Kharaziha et al. conducted proteomic analysis on EVs derived from prostate cancer cells sensitive vs. resistant to docetaxel, identifying multidrug resistance protein 1 (MDR-1), MDR-3, endophilin-A2, and poly(A) binding protein 4 as proteins enriched in the latter as well as present in EVs from the serum of castration-resistant prostate cancer patients, suggesting that EVs may be used as biomarker candidates for predicting therapeutic response or resistance[143].
Larger longitudinal studies will be crucial to validate the biomarkers identified thus far, but the field holds great promise.
Factors hindering the clinical application of liquid biopsies
Although liquid biopsies are extremely promising tools, there are issues that need to be addressed before widespread clinical adoption can occur. First, since the techniques used are extremely sensitive, even small differences at the sample collection or processing level can cause significant differences in the final outcome. The use of serum instead of plasma, for example, can increase the amount of cell-free DNA released from other sources such as leukocytes, thus reducing the diagnostic ability of NGS-based assays, especially when trying to detect rare variants[144]. Moreover, lifestyle-related factors can affect the release of cell-free DNA in the general circulation, thus constituting an array of possible confounding factors that are hard to identify and characterize systematically[145]. As for CTCs, they are usually extremely rare and hard to capture, and although the CellSearch method has provided the field with a standardized method, CTCs captured this way are not viable. This means they can only be used for DNA and FACS/Immunofluorescence studies, but not for RNA-based assays or functional assays, including patient-derived xenografts or in vitro drug sensitivity tests[146,147]. Moreover, CTC analysis has some of the drawbacks as classical biopsies, since they are not necessarily representative of the entire tumor, but only a subpopulation of cells that were able to migrate and survive in the circulation. A possible solution under investigation to at least partially solve this issue is choosing different sites for the blood collection. It has been reported that arterial blood and blood withdrawn from a closer site to the primary tumor may provide a higher number of CTCs[148,149]. Significant efforts have been made by societies in both the United States and Europe to draw standard guidelines for preanalytical sample treatment, but while the consensus is widely accepted, liquid biopsies remain technically challenging for both the clinician and the analytical lab staff, which need specific training and facilities that are not always available locally, as well as training to interpret the results correctly[150]. In addition to the general problems outlined above, EVs carry their own challenges at the preanalytical level. They are the most recently recognized source of biological information, and therefore their development as liquid biopsy tool is still at an early stage. A key example is the issue of EV isolation. There are several methods that are currently available to isolate EVs, namely differential ultracentrifugation, isopycnic ultracentrifugation, size-exclusion chromatography, polymer-based precipitation, immune-capture, asymmetric field flow fractionation, ultrafiltration, and the countless possible combinations among them[151]. Unfortunately, no technique is absolutely superior to another, and, depending on the one investigator’s use for EV isolation, the results obtained may vary[151]. Moreover, as stated above, EV secretion is stimulated by several factors that may relate to one’s lifestyle, which may make the detection of tumor-specific exosomes challenging[70]. Finally, while these considerations are valid for oncology in general, the field of bone oncology is currently lacking specific clinical trials, although we are confident that it is just a matter of time before this gap is filled.
DISCUSSION AND CONCLUSIONS
Liquid biopsies have the potential to become one of the most powerful instruments in the clinical oncologist’s toolkit, making diagnosis and prognosis increasingly accurate and therapy more personalized. In fact, the technique is rapidly gaining popularity, albeit still with somewhat limited success, as happens with most new implementations at early stages[39]. This is particularly important in bone oncology, where minimally invasive techniques to evaluate clinical response to therapy and prognosis are limited. Despite this, the field is currently lagging behind, and there is a significant gap that needs to be filled before concretely applying liquid biopsies in clinical practice. The potential benefits of liquid biopsies not only are related to the life quality and expectancy of cancer patients but also extend to the cost-effectiveness of treatments. In this regard, a cost-consequence analysis was recently conducted comparing the use of tissue biopsy alone vs. tissue biopsy-liquid biopsy combined diagnostic strategy, in NSCLC[152], where the application of the latter implied lower overall medical expenses for the healthcare system compared to tissue biopsy alone. Additionally, liquid biopsies not only are a potential means of diagnosis but also can be used as risk-profiling tools, which could be able to identify subjects that are at risk for bone metastases, so that preventive therapies can be initiated. Of course, CTCs have been the most widely used in preclinical and clinical practice thus far, gaining FDA approval for use in some metastatic tumor prognosis (i.e., colorectal, breast, and prostate cancer), mainly due to the development of well-standardized isolation and analytical techniques. Nevertheless, ctDNA and tumor-derived EVs may become even more important than CTCs in the future, especially for personalized medicine. Indeed, they are easier to detect and characterize and can genetically reflect the tumor as a whole, providing the potential for real-time monitoring of tumor progression and development of chemoresistance, as has also been proposed or demonstrated in other neoplasias[153-155]. Liquid biopsies could also be useful in tracking metastases before they become overt and therapy resistance by implementing them in standard follow-up protocols and analyzing the emergence of mutations that are important for therapy resistance and metastasis, as the pioneering work by the Bardelli group already demonstrated in colorectal carcinoma, although timelines may need to be readapted[153,154]. Moreover, EVs also play an active role in malignancy and chemoresistance, and targeting them could be a valuable tool in the oncologist’s toolkit, especially considering that some of the most commonly used EV secretion inhibitors are clinically approved for other conditions (manumycin A, D-pantethine, imipramine, tipifarnib, neticonazole, climbazole, ketoconazole, and triademenol)[156] and could be repurposed to reduce EV-induced chemoresistance. We believe this research area is largely underexplored and will see an increase of interest in the next few years, also considering the advances in next-generation sequencing that could eventually lead to a “single-EV sequencing”, which would really open a new avenue for the field[157].
DECLARATIONS
Authors’ contributionsDrafted the manuscript: Ucci A, Ponzetti M
Reviewed the manuscript: Ucci A, Rucci N, Ponzetti M
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by an AIRC investigator grant to NR (No.24823) and an AIRC fellowship for Italy to MP (No.25432) for salary.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
2. Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int 2015;2015:421746.
3. Siddiqui JA, Partridge NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology (Bethesda) 2016;31:233-45.
6. Capulli M, Paone R, Rucci N. Osteoblast and osteocyte: games without frontiers. Arch Biochem Biophys 2014;561:3-12.
7. Wang L, You X, Zhang L, Zhang C, Zou W. Mechanical regulation of bone remodeling. Bone Res 2022;10:16.
8. Cullinane DM. The role of osteocytes in bone regulation: mineral homeostasis versus mechanoreception. J Musculoskelet Neuronal Interact 2002;2:242-4.
10. Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer 2009;125:229-34.
11. Durfee RA, Mohammed M, Luu HH. Review of osteosarcoma and current management. Rheumatol Ther 2016;3:221-43.
12. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA A Cancer J Clinicians 2022;72:7-33.
13. Kundu ZS. Classification, imaging, biopsy and staging of osteosarcoma. Indian J Orthop 2014;48:238-46.
14. Misaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: a comprehensive review. SICOT J 2018;4:12.
15. Li S, Sun W, Wang H, Zuo D, Hua Y, Cai Z. Research progress on the multidrug resistance mechanisms of osteosarcoma chemotherapy and reversal. Tumour Biol 2015;36:1329-38.
16. Fanelli M, Tavanti E, Patrizio MP, et al. Cisplatin resistance in osteosarcoma: in vitro validation of candidate DNA repair-related therapeutic targets and drugs for tailored treatments. Front Oncol 2020;10:331.
17. Tu J, Huo Z, Gingold J, Zhao R, Shen J, Lee DF. The histogenesis of ewing sarcoma. Cancer Rep Rev 2017;1:10.15761/CRR.1000111.
18. Esiashvili N, Goodman M, Marcus RB Jr. Changes in incidence and survival of ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. J Pediatr Hematol Oncol 2008;30:425-30.
19. May WA, Grigoryan RS, Keshelava N, et al. Characterization and drug resistance patterns of Ewing’s sarcoma family tumor cell lines. PLoS One 2013;8:e80060.
20. Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with Ewing’s sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol 2015;39:189-95.
21. Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a children’s oncology group study. J Clin Oncol 2009;27:2536-41.
22. Bailey KM. Prospective investigation of drug resistance: an approach to understanding and optimizing the clinical benefit of targeted agents in Ewing sarcoma. Oncotarget 2018;9:37270-1.
24. Quiroz-Munoz M, Izadmehr S, Arumugam D, Wong B, Kirschenbaum A, Levine AC. Mechanisms of osteoblastic bone metastasis in prostate cancer: role of prostatic acid phosphatase. J Endocr Soc 2019;3:655-64.
25. Turpin A, Duterque-Coquillaud M, Vieillard MH. Bone metastasis: current state of play. Transl Oncol 2020;13:308-20.
27. Joeckel E, Haber T, Prawitt D, et al. High calcium concentration in bones promotes bone metastasis in renal cell carcinomas expressing calcium-sensing receptor. Mol Cancer 2014;13:42.
28. Ardura JA, Álvarez-Carrión L, Gutiérrez-Rojas I, Alonso V. Role of calcium signaling in prostate cancer progression: effects on cancer hallmarks and bone metastatic mechanisms. Cancers (Basel) 2020;12:1071.
29. Owyong M, Chou J, van den Bijgaart RJ, et al. MMP9 modulates the metastatic cascade and immune landscape for breast cancer anti-metastatic therapy. Life Sci Alliance 2019;2:e201800226.
30. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 2006;25:9-34.
31. Rucci N, Teti A. Osteomimicry: how the seed grows in the soil. Calcif Tissue Int 2018;102:131-40.
32. Capulli M, Hristova D, Valbret Z, et al. Notch2 pathway mediates breast cancer cellular dormancy and mobilisation in bone and contributes to haematopoietic stem cell mimicry. Br J Cancer 2019;121:157-71.
33. Pape F, Vargas G, Clézardin P. The role of osteoclasts in breast cancer bone metastasis. J Bone Oncol 2016;5:93-5.
35. Guise TA, Yin JJ, Taylor SD, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 1996;98:1544-9.
36. Kang J, La Manna F, Bonollo F, et al. Tumor microenvironment mechanisms and bone metastatic disease progression of prostate cancer. Cancer Lett 2022;530:156-69.
37. Yin JJ, Mohammad KS, Käkönen SM, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci USA 2003;100:10954-9.
38. Eslami-s Z, Cortés-hernández LE, Alix-panabières C. The metastatic cascade as the basis for liquid biopsy development. Front Oncol 2020;10:1055.
39. Palmirotta R, Lovero D, Cafforio P, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol 2018;10:1758835918794630.
40. Li X, Seebacher NA, Hornicek FJ, Xiao T, Duan Z. Application of liquid biopsy in bone and soft tissue sarcomas: Present and future. Cancer Lett 2018;439:66-77.
41. Joerger M, Huober J. Diagnostic and prognostic use of bone turnover markers. Recent Results Cancer Res 2012;192:197-223.
42. Singer FR, Eyre DR. Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med 2008;75:739-50.
43. Huang Q, Ouyang X. Biochemical-markers for the diagnosis of bone metastasis: a clinical review. Cancer Epidemiol 2012;36:94-8.
44. Kushlinskii NE, Babkina IV, Solov’ev YN, Trapeznikov NN. Vascular endothelium growth factor and angiogenin in the serum of patients with osteosarcoma and Ewing’s tumor. Bull Exp Biol Med 2000;130:691-3.
45. Krizkova S, Masarik M, Majzlik P, et al. Serum metallothionein in newly diagnosed patients with childhood solid tumours. Acta Biochim Pol 2010;57:561-6.
46. Markiewicz K, Zeman K, Kozar A, Gołebiowska-Wawrzyniak M. Evaluation of selected cytokines in children and adolescents with osteosarcoma at diagnosis - preliminary report. Med Wieku Rozwoj 2011:15,25-31.
47. Liu T, Ma Q, Zhang Y, et al. Self-seeding circulating tumor cells promote the proliferation and metastasis of human osteosarcoma by upregulating interleukin-8. Cell Death Dis 2019;10:575.
49. Wit S, van Dalum G, Terstappen LW. Detection of circulating tumor cells. Scientifica (Cairo) 2014;2014:819362.
50. Yang C, Xia BR, Jin WL, Lou G. Circulating tumor cells in precision oncology: clinical applications in liquid biopsy and 3D organoid model. Cancer Cell Int 2019;19:341.
51. Mauri G, Bonazzina E, Amatu A, et al. The evolutionary landscape of treatment for BRAFV600E mutant metastatic colorectal cancer. Cancers 2021;13:137.
52. Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med 2012;10:85.
53. Pellegrini C, Di Nardo L, Cipolloni G, et al. Heterogeneity of BRAF, NRAS, and TERT promoter mutational status in multiple melanomas and association with MC1R genotype: findings from molecular and immunohistochemical analysis. J Mol Diagn 2018;20:110-22.
54. Rashid FA, Bhat GH, Khan MS, Tabassum S, Bhat MH. Variations in
55. Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 2013;10:472-84.
56. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014;30:255-89.
57. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019;8:727.
58. Stevic I, Buescher G, Ricklefs FL. Monitoring therapy efficiency in cancer through extracellular vesicles. Cells 2020;9:130.
59. Green TM, Alpaugh ML, Barsky SH, Rappa G, Lorico A. Breast cancer-derived extracellular vesicles: characterization and contribution to the metastatic phenotype. Biomed Res Int 2015;2015:634865.
60. Taverna S, Giusti I, D’Ascenzo S, Pizzorno L, Dolo V. Breast cancer derived extracellular vesicles in bone metastasis induction and their clinical implications as biomarkers. Int J Mol Sci 2020;21:3573.
61. Loftus A, Cappariello A, George C, et al. Extracellular vesicles from osteotropic breast cancer cells affect bone resident cells. J Bone Miner Res 2020;35:396-412.
62. Cappariello A, Rucci N. Tumour-derived extracellular vesicles (EVs): a dangerous “Message in A Bottle” for bone. Int J Mol Sci 2019;20:4805.
63. Valencia K, Luis-Ravelo D, Bovy N, et al. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol 2014;8:689-703.
64. Ucci A, Cappariello A, Ponzetti M, et al. Anti-osteoblastogenic, pro-inflammatory and pro-angiogenic effect of extracellular vesicles isolated from the human osteosarcoma cell line MNNG/HOS. Bone 2021;153:116130.
65. Lan M, Zhu XP, Cao ZY, Liu JM, Lin Q, Liu ZL. Extracellular vesicles-mediated signaling in the osteosarcoma microenvironment: Roles and potential therapeutic targets. J Bone Oncol 2018;12:101-4.
66. Perut F, Roncuzzi L, Baldini N. The emerging roles of extracellular vesicles in osteosarcoma. Front Oncol 2019;9:1342.
67. Gassmann H, Schneider K, Evdokimova V, et al. Ewing sarcoma-derived extracellular vesicles impair dendritic cell maturation and function. Cells 2021;10:2081.
68. Pachva MC, Lai H, Jia A, Rouleau M, Sorensen PH. Extracellular vesicles in reprogramming of the ewing sarcoma tumor microenvironment. Front Cell Dev Biol 2021;9:726205.
69. Bebelman MP, Janssen E, Pegtel DM, Crudden C. The forces driving cancer extracellular vesicle secretion. Neoplasia 2021;23:149-57.
70. Ludwig N, Whiteside TL, Reichert TE. Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci 2019;20:4684.
71. Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA 2017;8:e1413.
72. Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017;14:531-48.
73. Incorvaia L, Castiglia M, Perez A, et al. Liquid biopsy in breast cancer. In Liquid biopsy in cancer patients Current Clinical Pathology; Cham: Humana Press; 2017. p. 77-84.
74. Olsson E, Winter C, George A, et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med 2015;7:1034-47.
75. Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015;7:302ra133.
76. Beaver JA, Jelovac D, Balukrishna S, et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin Cancer Res 2014;20:2643-50.
77. Pécuchet N, Zonta E, Didelot A, et al. Base-position error rate analysis of next-generation sequencing applied to circulating tumor DNA in non-small cell lung cancer: a prospective study. PLoS Med 2016;13:e1002199.
78. Jia J, Huang B, Zhuang Z, Chen S. Circulating tumor DNA as prognostic markers for late stage NSCLC with bone metastasis. Int J Biol Markers 2018;33:222-30.
79. Vandekerkhove G, Struss WJ, Annala M, et al. Circulating tumor DNA abundance and potential utility in de novo metastatic prostate cancer. Eur Urol 2019;75:667-75.
80. Bidard F, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014;15:406-14.
81. Moussavi-Harami SF, Wisinski KB, Beebe DJ. Circulating tumor cells in metastatic breast cancer: a prognostic and predictive marker. J Patient Cent Res Rev 2014;1:85-92.
82. De Giorgi U, Valero V, Rohren E, et al. Circulating tumor cells and bone metastases as detected by FDG-PET/CT in patients with metastatic breast cancer. Ann Oncol 2010;21:33-9.
83. Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 2013;31:539-44.
84. Cheng M, Liu L, Yang HS, Liu GF. Circulating tumor cells are associated with bone metastasis of lung cancer. Asian Pac J Cancer Prev 2014;15:6369-74.
85. Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63.
86. Shaffer DR, Leversha MA, Danila DC, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res 2007;13:2023-9.
87. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9.
88. Helo P, Cronin AM, Danila DC, et al. Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: concordance with CellSearch assay and association with bone metastases and with survival. Clin Chem 2009;55:765-73.
89. Bortolini Silveira A, Bidard FC, Tanguy ML, et al. Multimodal liquid biopsy for early monitoring and outcome prediction of chemotherapy in metastatic breast cancer. NPJ Breast Cancer 2021;7:115.
90. Kodahl AR, Ehmsen S, Pallisgaard N, et al. Correlation between circulating cell-free PIK3CA tumor DNA levels and treatment response in patients with PIK3CA-mutated metastatic breast cancer. Mol Oncol 2018;12:925-35.
91. Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013;368:1199-209.
92. Wang P, Bahreini A, Gyanchandani R, et al. Sensitive detection of mono- and polyclonal ESR1 mutations in primary tumors, metastatic lesions, and cell-free DNA of breast cancer patients. Clin Cancer Res 2016;22:1130-7.
93. Rossi G, Mu Z, Rademaker AW, et al. Cell-free DNA and circulating tumor cells: comprehensive liquid biopsy analysis in advanced breast cancer. Clin Cancer Res 2018;24:560-8.
94. Bhadresha KP, Patel M, Jain NK, Rawal RM. A predictive biomarker panel for bone metastases: liquid biopsy approach. J Bone Oncol 2021;29:100374.
95. Yang XR, Pi C, Yu R, et al. Correlation of exosomal microRNA clusters with bone metastasis in non-small cell lung cancer. Clin Exp Metastasis 2021;38:109-17.
96. Wang Y, Fang YX, Dong B, et al. Discovery of extracellular vesicles derived miR-181a-5p in patient’s serum as an indicator for bone-metastatic prostate cancer. Theranostics 2021;11:878-92.
97. Bryant RJ, Pawlowski T, Catto JW, et al. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer 2012;106:768-74.
98. Biggs CN, Siddiqui KM, Al-Zahrani AA, et al. Prostate extracellular vesicles in patient plasma as a liquid biopsy platform for prostate cancer using nanoscale flow cytometry. Oncotarget 2016;7:8839-49.
99. McKiernan J, Donovan MJ, Margolis E, et al. A prospective adaptive utility trial to validate performance of a novel urine exosome gene expression assay to predict high-grade prostate cancer in patients with prostate-specific antigen 2-10ng/mL at initial biopsy. Eur Urol 2018;74:731-8.
100. Barris DM, Weiner SB, Dubin RA, et al. Detection of circulating tumor DNA in patients with osteosarcoma. Oncotarget 2018;9:12695-704.
101. Shulman DS, Klega K, Imamovic-Tuco A, et al. Detection of circulating tumour DNA is associated with inferior outcomes in Ewing sarcoma and osteosarcoma: a report from the Children’s oncology group. Br J Cancer 2018;119:615-21.
102. Shukla NN, Patel JA, Magnan H, et al. Plasma DNA-based molecular diagnosis, prognostication, and monitoring of patients with
103. Hayashi M, Chu D, Meyer CF, et al. Highly personalized detection of minimal Ewing sarcoma disease burden from plasma tumor DNA. Cancer 2016;122:3015-23.
104. Zhang H, Gao P, Xiao X, et al. A liquid biopsy-based method for the detection and quantification of circulating tumor cells in surgical osteosarcoma patients. Int J Oncol 2017;50:1075-86.
105. Li M, Lu Y, Long Z, et al. Prognostic and clinicopathological significance of circulating tumor cells in osteosarcoma. J Bone Oncol 2019;16:100236.
106. Wu ZJ, Tan JC, Qin X, Liu B, Yuan ZC. Significance of circulating tumor cells in osteosarcoma patients treated by neoadjuvant chemotherapy and surgery. Cancer Manag Res 2018;10:3333-9.
107. Chalopin A, Tellez-Gabriel M, Brown HK, et al. Isolation of circulating tumor cells in a preclinical model of osteosarcoma: effect of chemotherapy. J Bone Oncol 2018;12:83-90.
108. Benini S, Gamberi G, Cocchi S, et al. Detection of circulating tumor cells in liquid biopsy from Ewing sarcoma patients. Cancer Manag Res 2018;10:49-60.
109. Schleiermacher G, Peter M, Oberlin O, et al. Increased risk of systemic relapses associated with bone marrow micrometastasis and circulating tumor cells in localized ewing tumor. J Clin Oncol 2003;21:85-91.
110. Hayashi M, Zhu P, McCarty G, et al. Size-based detection of sarcoma circulating tumor cells and cell clusters. Oncotarget 2017;8:78965-77.
111. Bao Q, Gong L, Wang J, Wen J, Shen Y, Zhang W. Extracellular vesicle RNA sequencing reveals dramatic transcriptomic alterations between metastatic and primary osteosarcoma in a liquid biopsy approach. Ann Surg Oncol 2018;25:2642-51.
112. Ma W, Zhang X, Chai J, Chen P, Ren P, Gong M. Circulating miR-148a is a significant diagnostic and prognostic biomarker for patients with osteosarcoma. Tumour Biol 2014;35:12467-72.
113. Allen-rhoades W, Kurenbekova L, Satterfield L, et al. Cross-species identification of a plasma microRNA signature for detection, therapeutic monitoring, and prognosis in osteosarcoma. Cancer Med 2015;4:977-88.
114. Wang SN, Luo S, Liu C, et al. miR-491 Inhibits Osteosarcoma Lung Metastasis and Chemoresistance by Targeting αB-crystallin. Mol Ther 2017;25:2140-9.
115. Yang Z, Zhang Y, Zhang X, et al. Serum microRNA-221 functions as a potential diagnostic and prognostic marker for patients with osteosarcoma. Biomed Pharmacother 2015;75:153-8.
116. Wang T, Ji F, Dai Z, Xie Y, Yuan D. Increased expression of microRNA-191 as a potential serum biomarker for diagnosis and prognosis in human osteosarcoma. Cancer Biomark 2015;15:543-50.
117. Zhou S, Wang B, Hu J, et al. miR-421 is a diagnostic and prognostic marker in patients with osteosarcoma. Tumour Biol 2016;37:9001-7.
118. Cong C, Wang W, Tian J, Gao T, Zheng W, Zhou C. Identification of serum miR-124 as a biomarker for diagnosis and prognosis in osteosarcoma. Cancer Biomark 2018;21:449-54.
119. Yao ZS, Li C, Liang D, et al. Diagnostic and prognostic implications of serum miR-101 in osteosarcoma. Cancer Biomark 2018;22:127-33.
120. Cai H, Zhao H, Tang J, Wu H. Serum miR-195 is a diagnostic and prognostic marker for osteosarcoma. J Surg Res 2015;194:505-10.
121. Nie CL, Ren WH, Ma Y, Xi JS, Han B. Circulating miR-125b as a biomarker of Ewing’s sarcoma in Chinese children. Genet Mol Res 2015;14:19049-56.
122. Samuel G, Crow J, Klein JB, et al. Ewing sarcoma family of tumors-derived small extracellular vesicle proteomics identify potential clinical biomarkers. Oncotarget 2020;11:2995-3012.
123. Russano M, Napolitano A, Ribelli G, et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: the potentiality of blood samples. J Exp Clin Cancer Res 2020;39:95.
124. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014;6:224ra24.
125. Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med 2015;7:313ra182.
126. O’Leary B, Hrebien S, Morden JP, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun 2018;9:896.
127. O’Leary B, Cutts RJ, Liu Y, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 2018;8:1390-403.
128. Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 2016;34:2961-8.
129. Galardi F, De Luca F, Biagioni C, et al. Circulating tumor cells and palbociclib treatment in patients with ER-positive, HER2-negative advanced breast cancer: results from a translational sub-study of the TREnd trial. Breast Cancer Res 2021;23:38.
130. Okegawa T, Itaya N, Hara H, Tambo M, Nutahara K. Circulating tumor cells as a biomarker predictive of sensitivity to docetaxel chemotherapy in patients with castration-resistant prostate cancer. Anticancer Res 2014;34:6705-10.
131. Liu W, Zhao X, Zhang YJ, Fang GW, Xue Y. MicroRNA-375 as a potential serum biomarker for the diagnosis, prognosis, and chemosensitivity prediction of osteosarcoma. J Int Med Res 2018;46:975-83.
132. Yuan J, Chen L, Chen X, Sun W, Zhou X. Identification of serum microRNA-21 as a biomarker for chemosensitivity and prognosis in human osteosarcoma. J Int Med Res 2012;40:2090-7.
133. Xavier CPR, Caires HR, Barbosa MAG, Bergantim R, Guimarães JE, Vasconcelos MH. The role of extracellular vesicles in the hallmarks of cancer and drug resistance. Cells 2020;9:1141.
134. Torreggiani E, Roncuzzi L, Perut F, Zini N, Baldini N. Multimodal transfer of MDR by exosomes in human osteosarcoma. Int J Oncol 2016;49:189-96.
135. Yoshida A, Fujiwara T, Uotani K, et al. Clinical and functional significance of intracellular and extracellular microRNA-25-3p in osteosarcoma. Acta Med Okayama 2018;72:165-74.
136. Wei F, Ma C, Zhou T, et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer 2017;16:132.
137. Yuwen D, Ma Y, Wang D, et al. Prognostic role of circulating exosomal miR-425-3p for the response of NSCLC to platinum-based chemotherapy. Cancer Epidemiol Biomarkers Prev 2019;28:163-73.
138. Chen WX, Wang DD, Zhu B, et al. Exosomal miR-222 from adriamycin-resistant MCF-7 breast cancer cells promote macrophages M2 polarization via PTEN/Akt to induce tumor progression. Aging (Albany NY) 2021;13:10415-30.
139. Kreger BT, Johansen ER, Cerione RA, Antonyak MA. The enrichment of survivin in exosomes from breast cancer cells treated with paclitaxel promotes cell survival and chemoresistance. Cancers (Basel) 2016;8:111.
140. Ciravolo V, Huber V, Ghedini GC, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol 2012;227:658-67.
141. Yang SJ, Wang DD, Li J, et al. Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene 2017;623:5-14.
142. Wang T, Ning K, Lu TX, et al. Increasing circulating exosomes-carrying TRPC5 predicts chemoresistance in metastatic breast cancer patients. Cancer Sci 2017;108:448-54.
143. Kharaziha P, Chioureas D, Rutishauser D, et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget 2015;6:21740-54.
144. Pittella-Silva F, Chin YM, Chan HT, et al. Plasma or serum: which is preferable for mutation detection in liquid biopsy? Clin Chem 2020;66:946-57.
145. Merker JD, Oxnard GR, Compton C, et al. Circulating tumor DNA Analysis in patients with cancer: american society of clinical oncology and college of american pathologists joint review. J Clin Oncol 2018;36:1631-41.
146. Wong KHK, Tessier SN, Miyamoto DT, et al. Whole blood stabilization for the microfluidic isolation and molecular characterization of circulating tumor cells. Nat Commun 2017;8:1733.
147. Fehm TN, Meier-Stiegen F, Driemel C, et al. Diagnostic leukapheresis for CTC analysis in breast cancer patients: CTC frequency, clinical experiences and recommendations for standardized reporting. Cytometry A 2018;93:1213-9.
148. Terai M, Mu Z, Eschelman DJ, et al. Arterial blood, rather than venous blood, is a better source for circulating melanoma cells. EBioMedicine 2015;2:1821-6.
149. Buscail E, Chiche L, Laurent C, et al. Tumor-proximal liquid biopsy to improve diagnostic and prognostic performances of circulating tumor cells. Mol Oncol 2019;13:1811-26.
150. Heidrich I, Ačkar L, Mossahebi Mohammadi P, Pantel K. Liquid biopsies: Potential and challenges. Int J Cancer 2021;148:528-45.
151. Brennan K, Martin K, FitzGerald SP, et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep 2020;10:1039.
152. Gancitano G, Ravasio R, Dionisi M, Cortinovis D. Cost-consequence analysis of three different diagnostic strategies in the first- and second-line treatment of locally advanced or metastatic non-small-cell lung cancer. Health Econ Ther 2018;19:1.
153. Siravegna G, Bardelli A. Blood circulating tumor DNA for non-invasive genotyping of colon cancer patients. Mol Oncol 2016;10:475-80.
154. Siravegna G, Bardelli A. Genotyping cell-free tumor DNA in the blood to detect residual disease and drug resistance. Genome Biol 2014;15:449.
155. Gutteridge A, Rathbone VM, Gibbons R, et al. Digital PCR analysis of circulating tumor DNA: a biomarker for chondrosarcoma diagnosis, prognostication, and residual disease detection. Cancer Med 2017;6:2194-202.
156. Catalano M, O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles 2020;9:1703244.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Ucci A, Rucci N, Ponzetti M. Liquid biopsies in primary and secondary bone cancers. Cancer Drug Resist 2022;5:541-59. http://dx.doi.org/10.20517/cdr.2022.17
AMA Style
Ucci A, Rucci N, Ponzetti M. Liquid biopsies in primary and secondary bone cancers. Cancer Drug Resistance. 2022; 5(3): 541-59. http://dx.doi.org/10.20517/cdr.2022.17
Chicago/Turabian Style
Ucci, Argia, Nadia Rucci, Marco Ponzetti. 2022. "Liquid biopsies in primary and secondary bone cancers" Cancer Drug Resistance. 5, no.3: 541-59. http://dx.doi.org/10.20517/cdr.2022.17
ACS Style
Ucci, A.; Rucci N.; Ponzetti M. Liquid biopsies in primary and secondary bone cancers. Cancer Drug Resist. 2022, 5, 541-59. http://dx.doi.org/10.20517/cdr.2022.17
About This Article
Copyright
Data & Comments
Data
 Cite This Article 5 clicks
Cite This Article 5 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.