Natural bioactive compounds: a potential therapeutic strategy to sensitize bladder cancer to cisplatin treatment?
Abstract
Bladder cancer (BC) is the tenth most common cancer, and its incidence is steadily rising worldwide, with the highest rates in developed countries. Neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy is the standard therapy for patients with muscle-invasive bladder cancer. However, less than 50% of patients initially respond to this treatment and nearly all of them eventually develop resistance, which is an important barrier to long-term survival. Therefore, there is an urgent need to understand the mechanisms of cisplatin resistance in BC and develop ways to counteract them. Several preclinical studies have demonstrated that naturally derived bioactive compounds, such as phytochemicals and flavonoids, can enhance the antitumor activity of cisplatin, with minimal side effects, by targeting different pathways involved in cisplatin sensitivity and resistance. However, their poor bioavailability has been one of the main problems for their successful introduction into clinical practice. At present, however, many new formulations with greatly increased bioavailability are under study in several clinical trials with encouraging results.
Keywords
Bladder cancer (BC) is a current clinical problem due to its high incidence, prevalence, and mortality[1,2]. At diagnosis, 70% of patients present with non-muscle-invasive BC, a highly recurrent tumor treated by transurethral resection followed by BCG instillation. However, a high proportion of patients have recurrence and progress to muscle-invasive BC (MIBC), which is also present in 30% of patients at diagnosis[3]. At present, the treatment of MIBC has several limitations. Cisplatin-based neoadjuvant chemotherapy followed by radical cystectomy is the standard therapy, with the highest level of evidence, but it is only effective in 25%-50% of patients and confers a limited survival benefit[4]. Moreover, this survival benefit is only observed in patients with pathological response, while the delay in cystectomy in those who do not respond has a negative prognostic impact, highlighting the need for predictive biomarkers to identify those patients likely to benefit from cisplatin-based neoadjuvant treatment[5]. In addition, adjuvant chemotherapy has been recommended for patients with high-risk MIBC, although randomized trials have not provided conclusive evidence on the impact of this approach[6-8]. Furthermore, nearly 50% of patients will develop metastatic disease after undergoing radical cystectomy[3], and platinum-based chemotherapy is considered the cornerstone of treatment for these patients as well[9]. Nevertheless, immunotherapy - especially immune checkpoint inhibitors (ICIs) - are potentially effective in metastatic BC (mBC)[10,11], although only 20%-30% of mBC patients respond and there are no reliable predictive biomarkers of response[12]. Importantly, with the approval of ICIs for the treatment of mBC, they are now being studied in the neoadjuvant setting for MIBC with promising results[13]. Thus, based on this evidence, it is clear that cisplatin still plays an essential role in the treatment of MIBC. However, less than 50% of patients initially respond, and nearly all of them eventually develop resistance, which represents an important barrier to long-term survival.
In this issue of Cancer Drug Resistance, Rajendran et al.[14] elegantly reviewed the mechanisms of cisplatin activity and resistance in BC and the potential of bioactive natural compounds, such as phytochemicals and flavonoids, to overcome this resistance and improve therapeutic response. As they pointed out[14], resistance to cisplatin is a complex, multifactorial process that can be attributed to specific mechanisms intrinsic to BC biology or to general mechanisms common to different tumor types or drug pharmacokinetics. Several factors have been associated with resistance to cisplatin, including decreased intracellular drug concentration mediated by drug efflux pumps, alterations in DNA repair genes, and increased drug cytosolic inactivation[15]. I fully support their argument[14] that a greater understanding of the mechanisms of cisplatin activity and resistance, as well as the identification of potential biomarkers of response, is required so that we can select patients likely to respond to cisplatin and to improve strategies to fight resistance mechanisms. One such strategy is based on targeting the effectors and pathways involved in chemoresistance with natural compounds that can potentially synergize with platinum drugs, such as cisplatin. The antitumor effects of natural products are generally attributed to suppression of cell proliferation and metastasis, induction of apoptosis, and stimulation of autophagy, with minimal side effects[16].
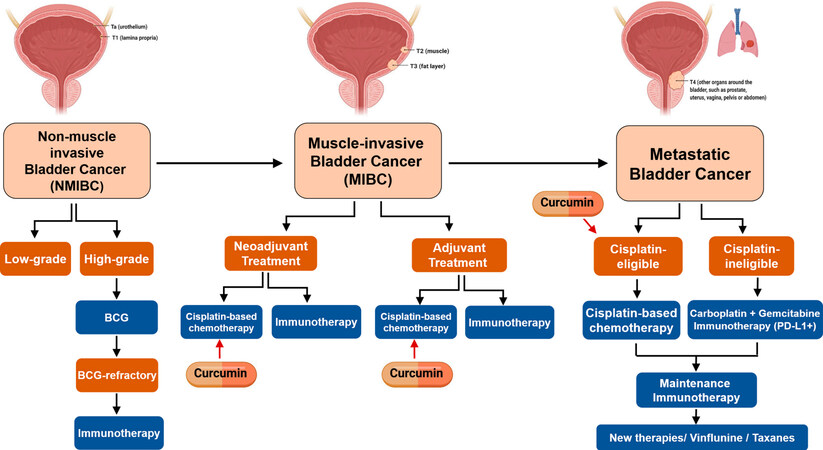
Based on our group’s experience in different tumor types, including mBC[17-19], we can highlight the potential and promising role of curcumin and its derivatives as chemosensitizing agents[20]. Curcumin
Figure 1. Potential clinical settings for the combined treatment of cisplatin plus curcumin in BC. Created with BioRender.com. BC: Bladder cancer.
However, although it is true that in vivo and in vitro data are promising, the clinical application of these naturally derived bioactive compounds has been limited by their poor bioavailability in humans due to poor absorption and water solubility as well as to their rapid metabolism and systemic elimination[25,26]. Nevertheless, to solve this problem, in recent years, many efforts have been focused on obtaining new promising bioavailable formulations or delivery strategies: liposomes, micelles, phospholipid complexes, microemulsions, nano-emulsions, emulsions, solid lipid nanoparticles, nanostructured lipid carriers, biopolymer nanoparticles, and microgels[27,28]. Nonetheless, as proposed by Rajendran et al.[14], high quality pharmacokinetic and pharmacodynamic studies are required for proper dosing of these new compounds. Moreover, increasing our understanding of how these compounds work is critical to developing synthetic derivatives with improved pharmacokinetics and greater bioavailability and efficacy.
In conclusion, although cisplatin is a clinical mainstay for the treatment of MIBC, many tumors unfortunately develop resistance and are refractory to treatment. As Rajendran et al.[14] pointed out, combination therapies using natural products represent a promising effective and novel strategy to overcome this resistance. For instance, the combination of curcumin and cisplatin could be a potential synergistic strategy to attenuate cisplatin-related adverse effects and decrease resistance. Finally, improved delivery strategies and new formulations offer encouraging ways to increase the bioavailability of these natural products. Hopefully, these strategies will be tested in further randomized, double-blind, placebo-controlled clinical trials in BC patients and incorporated into clinical practice. Nevertheless, although clinical trials using new bioavailable curcumin formulations are certainly mandatory to evaluate the optimal dosage, safety, and antitumor activity, based on our group’s previous preclinical results[19] as well as on those presented by Rajendran et al.[14], I suggest that the combination of curcumin plus a platinum agent, such as cisplatin, could be an effective and reliable approach for the management of several cancers, including BC.
DECLARATIONS
AcknowledgmentsI want to acknowledge Dr. Albert Font Pous for his scientific support and Renée Grupp for critical reading and language correction.
Authors’ contributionsThe author contributed solely to the article.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestThe author declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424.
3. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25-41.
4. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66.
5. Pardo JC, Ruiz de Porras V, Plaja A, et al. Moving towards personalized medicine in muscle-invasive bladder cancer: where are we now and where are we going? Int J Mol Sci 2020;21:6271.
6. Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol 2014;66:42-54.
7. Cognetti F, Ruggeri EM, Felici A, et al. Study Group(†). Adjuvant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: an Italian, multicenter, randomized phase III trial. Ann Oncol 2012;23:695-700.
8. Stadler WM, Lerner SP, Groshen S, et al. Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J Clin Oncol 2011;29:3443-9.
9. Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/ gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC intergroup study 30987. J Clin Oncol 2012;30:1107-13.
10. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67-76.
11. Bellmunt J, de Wit R, Vaughn DJ, et al. KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015-26.
12. Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol 2020;20:25-39.
13. Kaur J, Choi W, Geynisman DM, Plimack ER, Ghatalia P. Role of immunotherapy in localized muscle invasive urothelial cancer. Ther Adv Med Oncol 2021;13:17588359211045858.
14. Rajendran G, Taylor JA, Woolbrigh BL. Natural products as a means of overcoming cisplatin chemoresistance in bladder cancer. Cancer Drug Resist 2021;4:69-84.
15. Amable L. Cisplatin resistance and opportunities for precision medicine. Pharmacol Res 2016;106:27-36.
16. Sun CY, Zhang QY, Zheng GJ, Feng B. Phytochemicals: current strategy to sensitize cancer cells to cisplatin. Biomed Pharmacother 2019;110:518-27.
17. de Porras V, Layos L, Martínez-Balibrea E. Curcumin: a therapeutic strategy for colorectal cancer? Semin Cancer Biol 2021;73:321-30.
18. Font A, Ruiz de Porras V, Valderrama BP, et al. Epithelial-to-mesenchymal transition mediates resistance to maintenance therapy with vinflunine in advanced urothelial cell carcinoma. Cancers (Basel) 2021;13:6235.
19. Ruiz de Porras V, Bystrup S, Martínez-Cardús A, et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci Rep 2016;6:24675.
21. Park BH, Lim JE, Jeon HG, et al. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget 2016;7:63870-86.
22. Kao CC, Cheng YC, Yang MH, et al. Demethoxycurcumin induces apoptosis in HER2 overexpressing bladder cancer cells through degradation of HER2 and inhibiting the PI3K/Akt pathway. Environ Toxicol 2021;36:2186-95.
23. Abadi AJ, Mirzaei S, Mahabady MK, et al. Curcumin and its derivatives in cancer therapy: potentiating antitumor activity of cisplatin and reducing side effects. Phytother Res 2022;36:189-213.
24. Hussain Y, Islam L, Khan H, Filosa R, Aschner M, Javed S. Curcumin-cisplatin chemotherapy: a novel strategy in promoting chemotherapy efficacy and reducing side effects. Phytother Res 2021;35:6514-29.
25. Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 2014;46:2-18.
26. Cas M, Ghidoni R. Dietary curcumin: correlation between bioavailability and health potential. Nutrients 2019;11:2147.
27. Sohn SI, Priya A, Balasubramaniam B, et al. Biomedical applications and bioavailability of curcumin-an updated overview. Pharmaceutics 2021;13:2102.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Ruiz de Porras V. Natural bioactive compounds: a potential therapeutic strategy to sensitize bladder cancer to cisplatin treatment?. Cancer Drug Resist 2022;5:339-43. http://dx.doi.org/10.20517/cdr.2022.02
AMA Style
Ruiz de Porras V. Natural bioactive compounds: a potential therapeutic strategy to sensitize bladder cancer to cisplatin treatment?. Cancer Drug Resistance. 2022; 5(2): 339-43. http://dx.doi.org/10.20517/cdr.2022.02
Chicago/Turabian Style
Ruiz de Porras, Vicenç. 2022. "Natural bioactive compounds: a potential therapeutic strategy to sensitize bladder cancer to cisplatin treatment?" Cancer Drug Resistance. 5, no.2: 339-43. http://dx.doi.org/10.20517/cdr.2022.02
ACS Style
Ruiz de, Porras V. Natural bioactive compounds: a potential therapeutic strategy to sensitize bladder cancer to cisplatin treatment?. Cancer Drug Resist. 2022, 5, 339-43. http://dx.doi.org/10.20517/cdr.2022.02
About This Article
Copyright
Data & Comments
Data

 Cite This Article 5 clicks
Cite This Article 5 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.