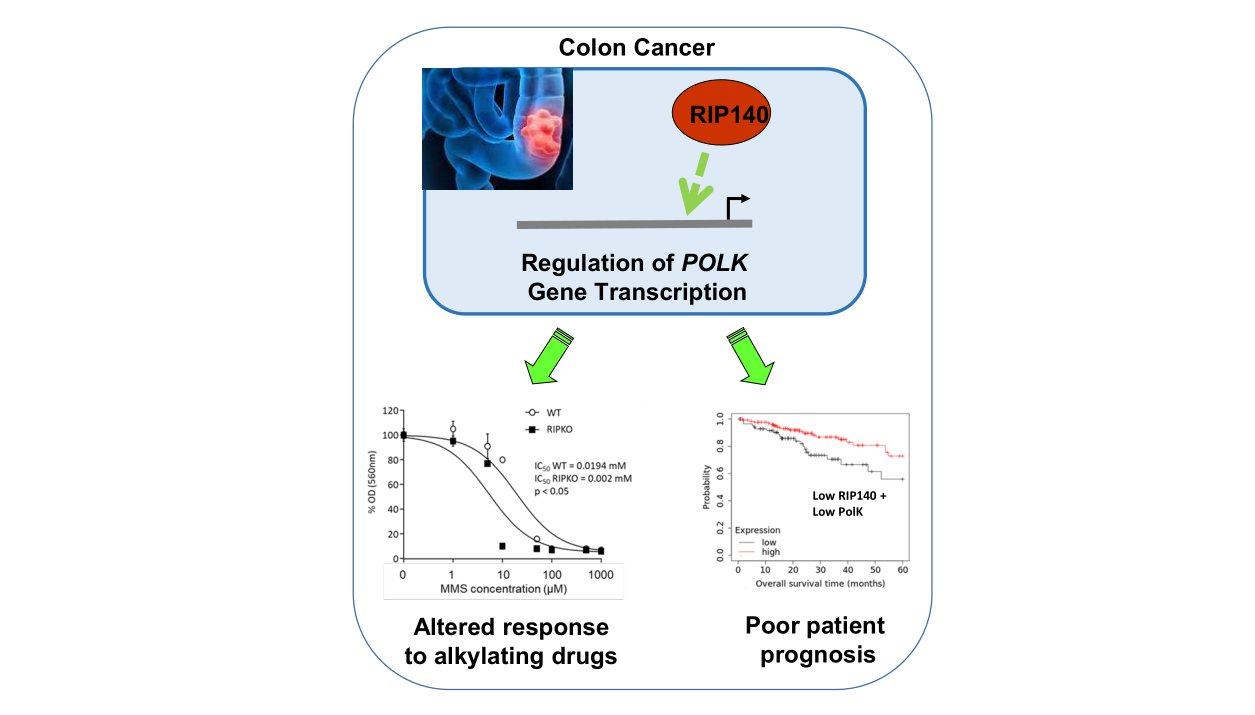
RIP140 regulates POLK gene expression and the response to alkylating drugs in colon cancer cells
Abstract
Aim: The transcription factor RIP140 (receptor interacting protein of 140 kDa) is involved in intestinal tumorigenesis. It plays a role in the control of microsatellite instability (MSI), through the regulation of MSH2 and MSH6 gene expression. The aim of this study was to explore its effect on the expression of POLK, the gene encoding the specialized translesion synthesis (TLS) DNA polymerase κ known to perform accurate DNA synthesis at microsatellites.
Methods: Different mouse models and engineered human colorectal cancer (CRC) cell lines were used to analyze by RT-qPCR, while Western blotting and luciferase assays were used to elucidate the role of RIP140 on POLK gene expression. Published DNA microarray datasets were reanalyzed. The in vitro sensitivity of CRC cells to methyl methane sulfonate and cisplatin was determined.
Results: RIP140 positively regulates, at the transcriptional level, the expression of the POLK gene, and this effect involves, at least partly, the p53 tumor suppressor. In different cohorts of CRC biopsies (with or without MSI), a strong positive correlation was observed between RIP140 and POLK gene expression. In connection with its effect on POLK levels and the TLS function of this polymerase, the cellular response to methyl methane sulfonate was increased in cells lacking the Rip140 gene. Finally, the association of RIP140 expression with better overall survival of CRC patients was observed only when the corresponding tumors exhibited low levels of POLK, thus strengthening the functional link between the two genes in human CRC.
Conclusion: The regulation of POLK gene expression by RIP140 could thus contribute to the maintenance of microsatellite stability, and more generally to the control of genome integrity.
Keywords
INTRODUCTION
Colorectal cancer (CRC) is one of the most frequent cancers worldwide and genetic instability exerts a driving role in this malignancy[1]. The mismatch repair (MMR) system is one of the various cellular systems involved in the maintenance of genome integrity through the correction of mistakes that occur during DNA replication. Once impaired (as occurs in 2%-3% of CRC cases with an inherited component including Lynch syndrome[2,3]), it generates microsatellite instability (MSI) and a hypermutated tumor phenotype with a high frequency of point and frameshift mutations[4,5].
In addition to molecular machineries that cope with DNA repair, mammalian cells possess enzymes with translesion DNA synthesis (TLS) activity. The POLK gene encodes Polκ, one of the Y-family TLS polymerases, which possesses unique DNA damage bypass capability[6]. Interestingly, despite any associated proofreading exonuclease activity, Polκ was shown to display high accuracy during dinucleotide microsatellite DNA synthesis[7], and Polκ-/- mice display a spontaneous mutator phenotype in various tissues[8]. This could be linked to its less open active site compared to other inaccurate specialized DNA polymerases from the Y family[9]. In fact, Polκ possesses a unique structural feature, an extension of its N-terminal region, called N-clasp, that protrudes from the thumb domain and encircles DNA in proximity to a primer terminus[10]. This results in a much more stable complex once Polκ binds to a mismatched DNA substrate, facilitating the extension step across various minor groove distortion or lesion. Besides its role in MSI maintenance together with MMR[7], Polκ seems to prevent DNA damage-induced toxic effects of methylnitrosourea, another process dependent on the MMR system[11]. This link between MMR and Polκ is reinforced by the findings that POLK interacts with MSH2[12] and partially protects human cells from the MMR-dependent cytotoxicity of O6-methylguanine lesions[11].
Our laboratory recently reported that the RIP140 (receptor interacting protein of 140 kDa) gene was involved in the normal and tumoral development of the intestinal epithelium. RIP140, also known as NRIP1 (nuclear receptor-interacting protein 1), was first identified as a transcriptional repressor of nuclear hormone receptors[13,14]. We and others then characterized RIP140 as a coregulator of various transcription factors, including, for instance, E2F[15] or NFKB[16]. The repressive activity of RIP140 involves several inhibitory domains interacting with histone deacetylases[17] and is controlled by different post-translational modifications[18]. Using a mouse model lacking the Rip140 gene, various physiological processes were shown to be regulated by RIP140, including female fertility[19] and mammary gland morphogenesis[20], fat metabolism[21], pro-inflammatory cytokine response[22], or cognition[23].
In the intestinal epithelium, our laboratory demonstrated that RIP140 inhibits the Wnt/β-catenin signaling pathway and, as a consequence, exerts an anti-proliferative effect[24]. In line with this result, we found that this transcription coregulator inhibits the Paneth cells lineage through the regulation of SOX9 expression and activity[25]. In addition, RIP140 expression decreased in CRC samples compared to the adjacent healthy tissue. In sporadic CRC, RIP140 mRNA and protein levels significantly correlated with better overall survival of patients and were identified as good prognosis markers[24]. More recently, we demonstrated that RIP140 was acting as a transcriptional regulator of MSH2 and MSH6 gene expression and was involved in the regulation of the MSI and hypermutator phenotype in CRC cells[26]. Interestingly, a frameshift mutation in the RIP140 coding sequence identified in MSI CRC tumors exhibits a dominant negative activity and correlates with shorter overall survival of patients with advanced CRC.
In the present study, we explored whether RIP140 could regulate POLK as a factor involved in microsatellite stability. We demonstrated that, in both engineered mouse models and human CRC cells, RIP140 positively regulates the POLK gene expression at the transcriptional level, at least partly via a p53-dependent mechanism. A strong correlation was observed between the expression of the RIP140 and POLK genes in CRC biopsies. Moreover, cells knocked out for the Rip140 gene were shown to be more sensitive to methyl methane sulfonate (MMS), a drug known to induce DNA lesions activating a POLK response. Finally, our data demonstrate a significant impact of POLK gene expression on the prognosis value of RIP140 in human CRC. We propose that the modulation of POLK gene expression by RIP140 could reinforce its effect on the maintenance of genome integrity and, more particularly, on the stability of microsatellite sequences.
METHODS
Plasmids
pRL-CMV-renilla and pGL promoters were obtained from Promega (Charbonnieres, France). pEF-cmyc-RIP140 was previously described[27]. pEGFP-RIP140 was a kind gift of Dr. Johanna Zilliacus, (Karolinska Institutet, Huddinge, Sweden)[28]. pEF-cmyc-RIPMSI was generated by mutagenesis using the QuikChange® Site-Directed Mutagenesis Kit (Stratagene). pEF-cmyc-RIPMSI was digested with AflII and EcoRV enzymes, and the resulting insert was cloned into pEGFP-RIP140 to create pEGFP-RIPMSI. GFP, GFP-RIP140, and GFP-RIPMSI were PCR amplified and cloned into pTRIPZ previously digested with AgeI and MluI to create pTRIPZ-GFP, pTRIPZ-RIP140 and pTRIPZ-RIPMSI, respectively. All the engineered PCR constructs were sequenced.
Cell culture and transfections
Mouse embryonic fibroblasts (MEFs) derived from wild-type or RIPKO mice previously described[24], and the stably transfected MEFs described by Palassin et al.[26] were grown in DMEM-F12 medium supplemented with 10% FCS, 100 U/mL penicillin, 100 mg/mL streptomycin, and 100 mg/mL sodium pyruvate, with 40 µg/mL puromycine for selection of stably transfected cells. HCT116, RKO, SW480, and HT29 human colon cancer cells were grown as previously described[26] and stably transfected with the empty pEGFP vector (Clontech®) or with the same vector containing the full-length human RIP140 cDNA. The SW620 human cell line was grown identically. HCT116-GFP and HCT116-RIP140 cells were previously described[24] and grown in McCoy medium and 750 μg/mL G418. Small interfering RNA (siRNA) transfections were performed using INTERFERin® on cells seeded the day before in a 6-well plate (3 × 105 cells per well). Each transfection was performed in triplicate, and interference efficiencies were tested by quantitative RT-PCR.
Animals
C57BL/6J Rip140-/- (RIPKO) mice[19] were given by Pr MG Parker (Imperial College London, London, UK). C57BL/6/129 RIP140 transgenic (RIPTg) mice were generated using the Speedy Mouse® Technology (Nucleis) by insertion of a single copy of the human RIP140 cDNA at the HPRT locus[24]. All animals were maintained under standard conditions, on a 12 h:12 h light/dark schedule and fed a chow diet ad libitum, according to European Union guidelines for the use of laboratory animals. In vivo experiments were performed in compliance with the French guidelines for experimental animal studies (agreement B34-172-27).
Luciferase assays
HCT116 cells were plated in 96-well plates (2.5 × 104 cells per well) 24 h prior to DNA transfection with Jet-PEI® (275 ng of total DNA). The pGL3-POLK Luc and its truncated mutant pGL3-83 vectors were previously described[29]. The pGL3-29 vector was constructed for this work and co-transfected in HCT116 cells. The pRL-CMV-renilla plasmid (Ozyme®) was used to normalize transfection efficiency. Firefly luciferase values were measured and normalized by the Renilla luciferase activity. Values were expressed as the mean ratio of luciferase activities.
Cell proliferation and cytotoxicity assays
Cells were seeded in quadruplicate at a density of 2 × 103 cells per well. At the indicated time, 0.5 mg/mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich®, St Louis, MO, USA) was added and incubated at 37 °C for 4 h. Formazan crystals were solubilized in DMSO and absorbance read at 560 nm on a spectrophotometer. The results were normalized to the cell density at Day 1. For cytotoxicity assays, cells were seeded in quadruplicate in a 96-well plate (2.5 × 103 cells per well) and exposed the day after to increasing concentrations of cytotoxic drugs orto vehicle alone. The cells were exposed to the drug during six days and cell proliferation was quantified each day using MTT assay. The results were normalized to the mean optical density of the control for each day. MMS and cisplatin were obtained from Sigma-Aldrich®.
Real-time quantitative PCR
Total RNA was extracted from cells using Quick-RNA kit (Zymo Research) according to the manufacturer’s instructions. Total RNA (1 µg) was subjected to reverse-transcription using qScript cDNA SuperMix (QuantaBio, VWR). Real-time quantitative PCR (RT-qPCR) were performed with the Roche LightCycler® 480 instrument and the PerfeCTa SYBR Green FastMix (QuantaBio, VWR) and were carried out in a final volume of 10 μL using 0.25 µL of each primer (25 μM), 5 μL of the supplied enzyme mix, 2.5 μL of H2O, and 2 μL of the template diluted at 1:10 [see Supplementary Table 1 for primer sequences]. After pre-incubation at 95 °C, runs corresponded to 35 cycles of 15 s each at 95 °C, 5 s at 60 °C, and 15 s at 72 °C. Melting curves of the PCR products were analyzed using LightCycler® software to exclude amplification of unspecific products. The results were normalized to different housekeeping gene transcripts (mouse RS9 or human 28S)[30].
Immunoblotting
RIPA solution was used to extract whole-cell proteins. Cell extracts were analyzed after migration of 30 µg protein extract by Western blotting using a primary polyclonal antibody against Polκ (1/1000, Abcam ab57070). Protein quantifications were normalized with the β-actin signal (1/1000, Millipore).
DNA microarray analysis
Published DNA microarray datasets, GSE39582[31] and GSE42284[32], were reanalyzed for RIP140 and POLK expression using the Cancertool database[33]. A transcriptomic dataset from the TCGA-COAD RNA-seq data was also used[4]. Another published DNA microarray study obtained on a cohort encompassing 396 colon tumor samples with MSS and MSI CRC[34] was also reanalyzed for RIP140 and POLK mRNA expression. Statistical significance was assessed using a Spearman correlation analysis. Correlation between RIP140 and POLK gene expression was also studied using another cohort[35]. RNA sequencing data from the TCGA[4] were reanalyzed using Cox proportional hazard regression[36]. RNAseq data obtained from CRC samples from the TCGA dataset were analyzed as described previously[37]. The Kaplan-Meier method was used to estimate overall survival (OS) calculated from the diagnosis until death. Patients lost to follow-up were censored at the time of last contact.
Statistical analysis
All experiments were conducted independently at least three times. The results were expressed as the mean ± standard deviation (S.D.). Statistical comparisons were performed with Mann-Whitney or Spearman tests. For the Cancertool database analysis, a Pearson’s test was performed for the correlation analyses. A probability level (P-value) of 0.05 was chosen for statistical significance.
RESULTS
RIP140 regulates POLK gene expression in mouse models
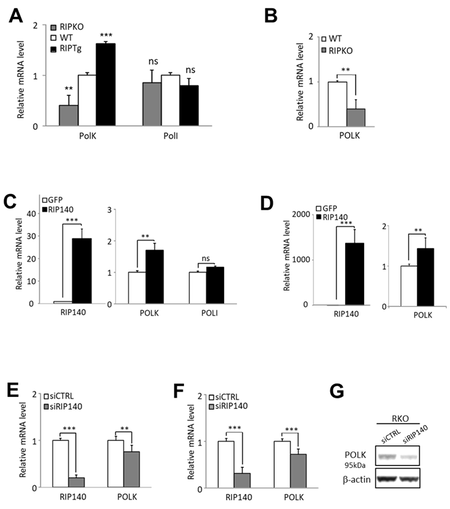
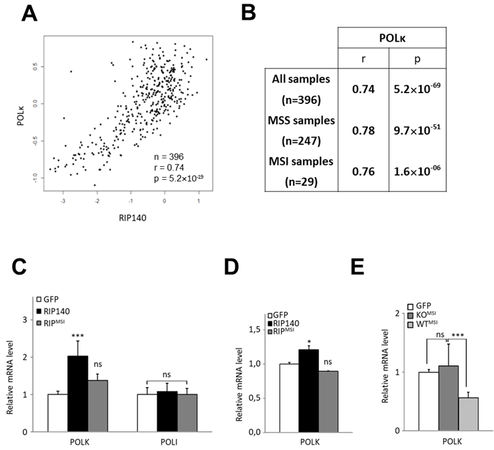
To explore the role of RIP140 on the regulation of the POLK polymerase gene expression, we first used transgenic mice in which the Rip140 gene was either knocked out (RIPKO mice) or overexpressed (RIPTg mice) [24]. As shown in Figure 1A, a significant decrease in the levels of Polκ mRNA was observed in the intestinal epithelium of RIPKO mice, whereas an increase was noted in RIPTg mice as compared to wild-type animals (WT). These regulations appeared specific since the expression of other TLS polymerase genes from the Y subfamily such as PolI did not vary between the different genotypes [Figure 1A and data not shown]. The steady-state levels of Polκ mRNA were also significantly reduced in immortalized MEFs derived from the RIPKO mice as compared to cells isolated from WT animals [Figure 1B]. Altogether, these results demonstrate that RIP140 positively controls the expression of the Polκ gene in mouse cells and tissues.
Figure 1. RIP140 regulates POLK gene expression. (A) RT-qPCR analysis of Polκ and PolI mRNA in the intestinal epithelium of mice lacking the Rip140 gene (RIPKO) and in transgenic mice overexpressing RIP140 (RIPTg) as compared to their WT littermates. The results for each gene are given in arbitrary units (AU) and expressed in fold change ± S.D. relative to WT after normalization to mouse RS9 mRNA. (B) The same as in (A) in immortalized MEFs from WT or RIPKO mice. (C) HCT116 cells were stably transfected with the pEGFP-RIP140-expressing vector (HCT-RIP140) or pEGFP alone (HCT-GFP). RIP140, POLK, and POLI mRNA levels were quantified by RT-qPCR. The results are expressed relative to GFP cells after normalization to human 28S mRNA. (D) The same as in (C) in HCT116 cells transiently transfected with a RIP140 expression vector. (E) RIP140 and POLK mRNA levels were quantified by RT-qPCR in HCT116 cells transiently transfected with siCTRL or siRIP140 siRNAs as indicated. The results are expressed as fold change ± S.D. relative to siCTRL after normalization to 28S mRNA (n = 3 independent experiments for each condition). (F) The same as in (E) performed in RKO CRC cells. (G) POLK expression analysis by Western blot of whole cell extract from RKO cells 48 h after transient siRNA transfection. Quantifications are expressed in AU after normalization to β-actin, used as a control of protein migration. A Mann-Whitney test was used for statistical analysis (ns = not significant, **P < 0.01, ***
RIP140 regulates POLK gene expression in CRC cells
To check whether this regulation can be observed in human cells, we then analyzed the effect of RIP140 on POLK gene expression in human CRC cell lines. We confirmed the increased expression of the POLK gene at the mRNA levels in HCT116 cells either stably overexpressing RIP140 [Figure 1C] or transiently transfected with a RIP140 expression vector [Figure 1D]. We also unveiled that silencing the expression of the RIP140 gene in HCT116, RKO or HCT29 CRC cells significantly affected POLK mRNA abundance
Transcriptional regulation of POLK gene transcription in CRC cells
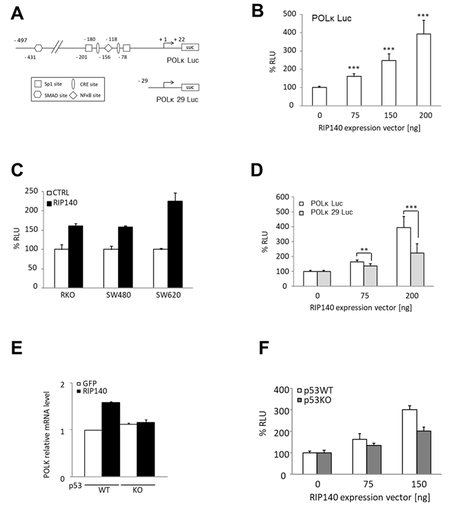
To decipher the mechanisms underlying the positive regulation of POLK gene expression by RIP140, we set up transient transfection experiments of HCT116 cells using the POLK Luc reporter construct encompassing the proximal promoter region of the POLK gene [Figure 2A]. As observed in Figure 2B, RIP140 significantly increased, in a dose-dependent manner, the luciferase activity driven by the POLK gene promoter, thus supporting a positive transcriptional regulation by RIP140. The same effects were observed in other CRC cells including RKO, SW480, and SW620 cells [Figure 2C].
Figure 2. RIP140 regulates POLK at the transcriptional level in CRC cells. (A) Schematic representation of POLK proximal promoter cloned in a pGL3 promoter and the putative regulatory sites identified as well as the POLK 29 Luc deletion mutant. (B) The POLK Luc construct was transiently co-transfected into HCT116 cells with increasing doses of a pEF-cmyc-RIP140 expression vector and pRL-CMV-renilla as an internal control. The reporter activity is presented as relative luciferase activity (RLU) as mean ± S.D. (n = 3 independent experiments). (C) The same reporter assay as in (B) performed in RKO, SW480, and SW620 cells. (D) Luciferase reporter assay performed with the two reporter vectors described in (A), in HCT116 cells. Values, expressed as a percent of control, are means ± S.D. (n = 3 independent experiments). (E) The effect of RIP140 ectopic expression on POLK mRNA levels after transient transfection in p53WT or p53KO HCT116 cells. (F) Transactivation of the POLK gene promoter by increasing doses of a RIP140 expression vector in p53WT or p53KO HCT116 cells. A Mann-Whitney test was used for statistical analysis (**P < 0.01 and ***P < 0.001).
Mechanism of the transcriptional regulation by RIP140
Although RIP140 was first identified as a transcriptional repressor, we and others reported positive regulation of gene expression (for a review, see Ref.[38]). In particular, we described a transcriptional activation of gene expression by RIP140 through Sp1-mediated mechanisms[39]. Since the POLK gene promoter exhibits Sp1 binding sites, we tested if the deletion of these sites affected the transcriptional response to RIP140 ectopic expression. As shown in Figure 2D, the induction of luciferase activity by RIP140 was significantly reduced when we used a short reporter construct (POLK 29 Luc), suggesting that the regulation of POLK gene expression by RIP140 might be, at least in part, mediated by Sp1.
Interestingly, a close correlation between elevated POLK expression and p53 inactivation was previously reported in lung cancer tissues[40]. This effect is likely due to the direct role of p53 on the POLK gene since p53 strongly inhibits the POLK promoter activity in lung cancer cells, while this activity is much higher in p53-/- MEF than in p53+/- and p53+/+ MEFs[40]. Therefore, we checked whether the positive regulation of POLK gene expression by RIP140 was dependent on p53. By using HCT116 cells expressing or not the TP53 gene, we found that, indeed, the regulation of the POLK gene by RIP140 was abolished in HCT116 p53-/- cells when monitored at the mRNA level [Figure 2E] or using a luciferase reporter assay [Figure 2F], supporting that p53 is involved in the RIP140-mediated regulation of POLK levels.
Correlation of gene expression in human CRC biopsies
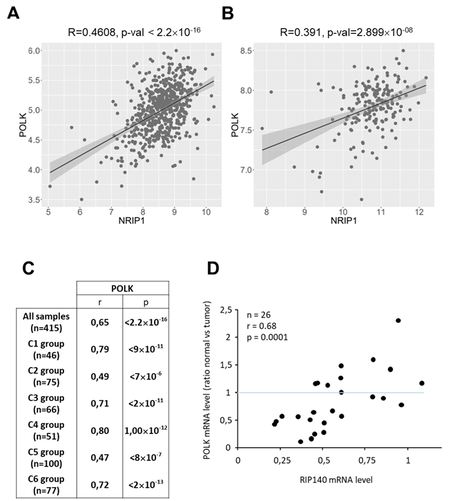
To validate in human CRC biopsies the expression data obtained in mouse tissues and in CRC cells in vitro, we used the Cancertool database[33] to reanalyze the published Affymetrix DNA microarray data from two cohorts, namely GSE39582 containing 585 tumors[31] and GSE42284 with 188 samples[32]. As shown in Figure 3A and B, and in perfect agreement with the data from mice, the results clearly show a significant positive correlation of RIP140 mRNA levels with those of POLK in CRC biopsies. We also reanalyzed another transcriptomic dataset from the TCGA-COAD RNA-seq data[4] obtained on 415 samples, which confirmed a significant correlation between RIP140 and POLK mRNA levels (r = 0.65; P < 2.2 × 10-16) [Figure 3C]. This dataset was further reanalyzed, taking into account the different molecular subtypes[31]. This allowed us to show a significant correlation of RIP140 gene expression with that of POLK in the six different subgroups, in particular in the C2 group which corresponds to the MMR deficient subgroup. Finally, we confirmed these data in another cohort comparing the expression of “DNA replication” genes in CRC and in the adjacent normal mucosa[35]. We confirmed that RIP140 gene expression strongly decreased in the tumor (data not shown) and that there was a strong correlation in the expression ratio (normal vs. tumoral) of the two genes (r = 0.68; P = 0.0001) [Figure 3D].
Figure 3. Correlation of RIP140 and POLK gene expression in human CRC samples. (A) Analysis of RIP140 and POLK correlation using the mRNAs expression from the Cancertool database in the GSE39582 cohort[31]. The correlation coefficient and the p-value (Pearson’s test) are indicated. (B) Same as in (A) with the cohort GSE42284[32]. (C) Correlations between RIP140 and POLK gene expression performed with TCGA RNA-Seq cohort (n = 415)[4] showing the correlations found in the different CRC molecular subtypes described in this cohort. (D) Correlation between POLK and RIP140 gene expression in a cohort comparing the expression of “DNA replication” genes in CRC and in the adjacent normal mucosa (n = 26)[35].
Effect of the RIPMSI mutation on the regulation of POLK gene expression
We recently demonstrated that RIP140 plays an important role in controlling microsatellite instability through the regulation of MSH2 and MSH6 genes[26]. To compare the correlation between RIP140 and POLK gene expression in microsatellite stable (MSS) and instable (MSI) CRC tumors, we reanalyzed a transcriptomic dataset from 396 human CRC with both types of tumors[34]. As shown in Figure 4A, we observed a very significant positive correlation between RIP140 and POLK mRNA levels in the whole cohort (r = 0.74; P = 5.2 × 10-69), thus confirming the results shown in Figure 3. Interestingly, POLK and RIP140 gene expression were significantly correlated in both MSS and MSI tumors [Figure 4B].
Figure 4. RIP140 and POLK in MSI CRC tumors. (A) Correlation between RIP140 and POLK gene expression in 396 colorectal adenocarcinomas[34]. (B) Statistical significance was assessed using a Spearman correlation analysis on this cohort, containing 247 microsatellite stable (MSS) and 29 microsatellite instable (MSI) samples. Spearman correlation coefficients between RIP140 and POLK gene expression are indicated for the whole cohort, as well as MSS and MSI samples. (C) mRNA quantification of the POLK and POLI genes in MEF RIPKO stable cells expressing either the GFP (white box) or the GFP fused wild-type form (black box) or the RIPMSI mutant form (grey box) of RIP140. (D) mRNA quantification of the POLK gene in HT29 CRC cells transiently transfected with pEGFP, pEGFP-RIP140, or pEGFP-RIPMSI expression vectors. (E) Analyses of the mRNA expression of the POLK gene in MEFs cells stably transfected with the human expression vector of RIPMSI in a RIP140 wild-type background (MEF WT) or knock-out (MEF RIPKO) as compared to the control transfected with a GFP expressing vector in each condition. A Mann-Whitney test was used for statistical analysis (ns = not significant, *P < 0.05, **P < 0.01 and ***P < 0.001).
In MSI CRC, we identified a frameshift mutation in the RIP140 coding sequence[26]. This mutation (called RIPMSI) generated a truncated protein which impaired the biological activity of the RIP140 protein. As shown in Figure 4C, the RIPMSI protein was found to be less efficient than the WT protein to increase POLK gene expression. The same results were obtained on endogenous gene expression in HT29 CRC cells overexpressing wild-type or mutated RIP140 [Figure 4D]. Interestingly, the RIPMSI mutant exhibited a dominant negative effect since its ectopic expression significantly decreased POLK mRNA accumulation only in WT MEFs, which express normal levels of RIP140, and not in RIPKO MEFs, which no longer express the Rip140 gene [Figure 4E].
Functional consequences of the regulation of POLK by RIP140
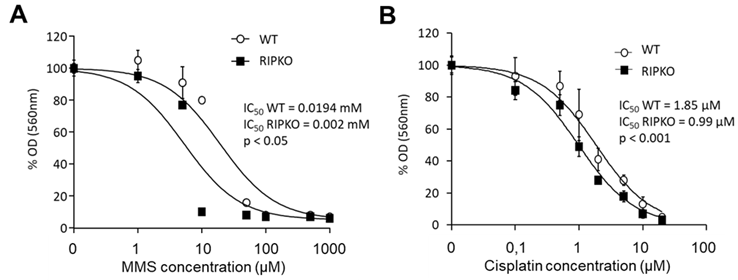
Since the POLK gene is involved in the cellular response to cytotoxic drugs including MMS and cisplatin through a TLS replication process[41], we next measured the importance of RIP140 on MMS and cisplatin sensitivity by comparing IC50 ratios between RIPKO cells and their WT counterparts. As shown in Figure 5A and B, we observed a significant increase of sensitivity to MMS and cisplatin when the RIP140 gene was knocked out (IC50 ratio WT/RIPKO = 9.7, P < 0.05 for MMS and IC50 ratio WT/RIPKO = 1.9,
Figure 5. RIP140 expression affects the response to cytotoxic drugs. (A) MEF WT and RIPKO cells were exposed or not to increasing doses of methyl methane sulfonate (from 0.5 µM to 1 mM). The optical density of diluted formazan crystals was expressed in percentage relative to the control. IC50 values of each cell type are mentioned together with the p-value of the nonlinear regression performed with GraphPad® software, allowing the comparison of IC50 between each cell type dose-response (IC50 ratio WT/RIPKO = 9.7, P < 0.05). (B) The same as in (A) with increasing doses of cisplatin (from 0.1 to 20 µM) (IC50 ratio WT/RIPKO = 1.9, P < 0.001). Mouse embryonic fibroblasts wild-type.
POLK expression impacts the prognosis value of RIP140 in CRC tumors
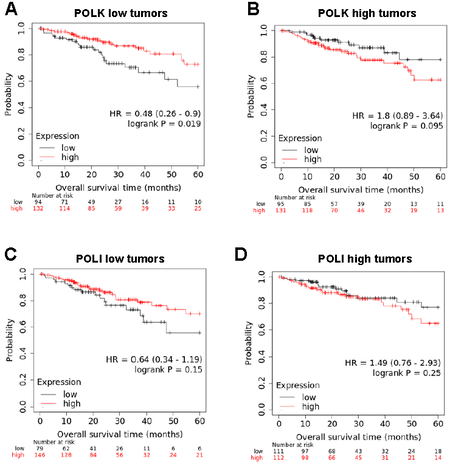
Using the Kaplan-Meier Plotter database, we then investigated whether POLK levels contribute to the good prognosis value of RIP140 that we previously reported in CRC patients[24]. We reanalyzed RNAseq data obtained from 452 patients with colon adenocarcinoma that we separated into two groups of 226 patients, each with low and high POLK gene expression in the corresponding tumors using the median as a cutoff value. As shown in Figure 6A and B, a statistically significant association of high expression of RIP140 with a decreased risk of death in CRC patients was observed when their tumor exhibited low POLK gene expression [Figure 6A] but not in tumors with high POLK gene expression [Figure 6B]. As a control, no differences were observed when the 452-patient cohort was stratified based on the median value of POLI expression [Figure 6C and D]. Collectively, all the data obtained in this work strongly support a molecular and functional link between RIP140 and POLK in CRC.
Figure 6. The prognosis value of RIP140 in CRC tumors is dependent on POLK gene expression. (A) Kaplan-Meier analysis of the cumulative OS of patients with low POLK gene expression was performed into the groups exhibiting low or high RIP140 gene expression. A log-rank test was used for statistical analysis. (B) The same analysis as in (A) with tumors expressing high POLK gene expression. (C,D) A similar study was performed using POLI levels to define the two subcohorts.
DISCUSSION
Colorectal cancer is a frequent neoplasm with high genomic instability including MSI due to defects in the MMR system. We previously described that the transcription factor RIP140 was a key factor in the regulation of intestinal homeostasis and tumorigenesis[24]. We also showed that RIP140 could be involved in microsatellite stability through the regulation of MSH2/MSH6 gene expression[26].
In the present study, we demonstrated that RIP140 also strongly regulates POLK gene expression, in both mouse and human cells and tissues. Data obtained using luciferase reporter assays demonstrate that this regulation took place at the transcriptional level and implicated the proximal region of the POLK gene. Several transcription factors including p53[40], Sp1, and CREB[29] or AhR[42] have been shown to control the expression of the POLK gene, and some of them have their activity regulated by RIP140[43]. The data presented herein suggest that p53 is a good candidate to mediate, at least partly, the regulation of POLK gene expression since the positive effect of RIP140 is lost in HCT116 cells no longer expressing the TP53 gene. Moreover, it has been shown that the HDAC inhibitor Trichostatin A was able to induce POLK gene expression[29]. Since RIP140 strongly interacts with HDACs[44], it is possible that HDAC sequestration out of the POLK gene promoter also participates in the positive effect exerted by RIP140 on POLK gene transcription as already demonstrated for other transcription factors[39]. Further work will be necessary to fully decipher the mechanisms used by RIP140 to transcriptionally control POLK gene expression.
Cell survival relies on a subtle equilibrium between accurate genomic DNA replication and less stringent DNA synthesis often linked to DNA damage. POLK plays an important and critical role in such equilibrium by contributing to several important DNA transaction pathways. These include translesion synthesis, which allows stalled replication forks to bypass a lesion and restart[45]; the replication checkpoint[46], a crucial pathway that regulates the replication stress response to a large array of insults that cause replication arrest; and the synthesis of intrinsic non-B microsatellite DNA[7]. Consistent with these multiple and complex roles, both overexpression of POLK (associated with advanced disease stages and shorter survival in patients with glioma[47] and non-small cell lung carcinomas[48]) and under-expression of POLK (frequently observed in colorectal, lung, stomach, and breast cancers[29,35,49,50]) have been documented to lead to genetic instability. Indeed, excessive Polκ can interfere with fork progression and trigger a mutator phenotype and chromosome instability, while downregulation of Polκ expression can affect fork progression and induce replicative stress. Since both situations can confer a selective growth advantage during cancer cell evolution, this underlines the importance of tight regulation of POLK gene expression at the transcriptional level for the maintenance of genome integrity. Therefore, our discovery here establishing the role of RIP140 in POLK gene regulation not only may explain why POLK is abnormally expressed in cancer, notably in CRC, but can also clarify why cancers cells respond differentially to anticancer genotoxic drugs that target DNA replication forks. This last point is illustrated in the present work by cell sensitivity to MMS treatment, which can be modulated by RIP140.
In addition, it is worth mentioning that the regulation of POLK gene expression could intensify the phenotypic consequences of the regulation of MutSα gene expression by RIP140[26]. Indeed, this TLS polymerase displays a high accuracy during dinucleotide microsatellite DNA synthesis and has been proposed to play a role in the maintenance of microsatellite stability[7]. Moreover, Polκ interacts with MSH2[12] and could therefore participate in the control of MutSα activity. Defining the precise involvement of POLK gene induction in the effect of RIP140 on the maintenance of genome integrity in colon cancer cells (in particular on the stability of microsatellites) and on their resistance to anticancer drugs will obviously require further work. It would also be interesting to decipher whether, as it is suggested for the MSH2 protein[51], POLK could be involved in the RIP140-mediated transcriptional regulation of gene expression.
Altogether, the present data suggest that POLK-defective cells exhibit an altered DNA replication program, thus explaining the spontaneous genetic alterations observed in POLK-deficient mice[8]. Transcriptional deregulation of POLK gene expression may therefore participate in intestinal tumorigenesis and account, at least in part, for the tumor suppressor role of RIP140 that we previously suggested.
DECLARATIONS
Authors’ contributionsSubstantial contributions to conception and design of the study: Cavaillès V, Castet-Nicolas A
Data acquisition, as well as technical and material support: Palassin P, Bonnet S, Lapierre M, Jalaguier S, Teyssier C, Pillaire MJ, Hoffmann JS
Data analysis, statistical analysis and interpretation: Palassin P, Lapierre M, Cavaillès V, Castet-Nicolas A
Bioinformatic analysis: Győrffy B
Manuscript writing: Palassin P, Lapierre M, Hoffmann JS, Cavaillès V, Castet-Nicolas A
The manuscript draft was inserted in the P. Palassin PhD thesis, and not published elsewhere
Manuscript reviewing and editing: Pillaire MJ, Jalaguier S, Teyssier C
Study supervision: Cavaillès V, Castet-Nicolas A
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by INSERM, INCa (Plan Cancer pour la Formation à la Recherche Translationnelle en Cancérologie; ASC14080FSA), CHU Montpellier (Contrat année recherche for PP), the Fondation Val d’Aurelle, Université de Montpellier 1 and the Institut régional du Cancer de Montpellier (ICM). We thank the Réseau d’Histologie Expérimentale de Montpellier (RHEM) for histology facilities.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateIn vivo experiments were performed in compliance with the French guidelines for experimental animal studies (agreement B34-172-27 for IRCM and CEEA-LR-12158 for the experiments, obtained from the ethics committee for animal experimentation).
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108.
2. Peltomäki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology 1997;113:1146-58.
3. Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3’ exons of TACSTD1. Nat Genet 2009;41:112-7.
4. Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7.
5. Carethers JM, Stoffel EM. Lynch syndrome and lynch syndrome mimics: the growing complex landscape of hereditary colon cancer. World J Gastroenterol 2015;21:9253-61.
7. Hile SE, Wang X, Lee MY, Eckert KA. Beyond translesion synthesis: polymerase κ fidelity as a potential determinant of microsatellite stability. Nucleic Acids Res 2012;40:1636-47.
8. Stancel JN, McDaniel LD, Velasco S, et al. Polk mutant mice have a spontaneous mutator phenotype. DNA Repair (Amst) 2009;8:1355-62.
9. Washington MT, Carlson KD, Freudenthal BD, Pryor JM. Variations on a theme: eukaryotic Y-family DNA polymerases. Biochim Biophys Acta 2010;1804:1113-23.
10. Lone S, Townson SA, Uljon SN, et al. Human DNA polymerase kappa encircles DNA: implications for mismatch extension and lesion bypass. Mol Cell 2007;25:601-14.
11. Lupari E, Ventura I, Marcon F, et al. Pol kappa partially rescues MMR-dependent cytotoxicity of O6-methylguanine. DNA Repair (Amst) 2012;11:579-86.
12. Lv L, Wang F, Ma X, et al. Mismatch repair protein MSH2 regulates translesion DNA synthesis following exposure of cells to UV radiation. Nucleic Acids Res 2013;41:10312-22.
13. Cavailles V, Dauvois S, L’Horset F, et al. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J 1995;14:3741-51.
14. Augereau P, Badia E, Carascossa S, et al. The nuclear receptor transcriptional coregulator RIP140. Nucl Recept Signal 2006;4:e024.
15. Docquier A, Harmand PO, Fritsch S, et al. The transcriptional coregulator RIP140 represses E2F1 activity and discriminates breast cancer subtypes. Clin Cancer Res 2010;16:2959-70.
16. Zschiedrich I, Hardeland U, Krones-Herzig A, et al. Coactivator function of RIP140 for NFkappaB/RelA-dependent cytokine gene expression. Blood 2008;112:264-76.
17. Castet A, Boulahtouf A, Versini G, et al. Multiple domains of the receptor-interacting protein 140 contribute to transcription inhibition. Nucleic Acids Res 2004;32:1957-66.
18. Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell 2008;31:449-61.
19. White R, Leonardsson G, Rosewell I, et al. The nuclear receptor co-repressor nrip1 (RIP140) is essential for female fertility. Nat Med 2000;6:1368-74.
20. Nautiyal J, Steel JH, Mane MR, et al. The transcriptional co-factor RIP140 regulates mammary gland development by promoting the generation of key mitogenic signals. Development 2013;140:1079-89.
21. Leonardsson G, Steel JH, Christian M, et al. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci U S A 2004;101:8437-42.
22. Ho PC, Tsui YC, Feng X, Greaves DR, Wei LN. NF-κB-mediated degradation of the coactivator RIP140 regulates inflammatory responses and contributes to endotoxin tolerance. Nat Immunol 2012;13:379-86.
23. Duclot F, Lapierre M, Fritsch S, et al. Cognitive impairments in adult mice with constitutive inactivation of RIP140 gene expression. Genes Brain Behav 2012;11:69-78.
24. Lapierre M, Bonnet S, Bascoul-Mollevi C, et al. RIP140 increases APC expression and controls intestinal homeostasis and tumorigenesis. J Clin Invest 2014;124:1899-913.
25. Gleizes A, Triki M, Bonnet S, et al. RIP140 represses intestinal paneth cell differentiation and interplays with SOX9 signaling in colorectal cancer. Cancers (Basel) 2021;13:3192.
26. Palassin P, Lapierre M, Pyrdziak S, et al. A truncated NRIP1 mutant amplifies microsatellite instability of colorectal cancer by regulating MSH2/MSH6 expression, and is a prognostic marker of stage III tumors. Cancers (Basel) 2021;13:4449.
27. Jalaguier S, Teyssier C, Nait Achour T, et al. Complex regulation of LCoR signaling in breast cancer cells. Oncogene 2017;36:4790-801.
28. Zilliacus J, Holter E, Wakui H, et al. Regulation of glucocorticoid receptor activity by 14--3-3-dependent intracellular relocalization of the corepressor RIP140. Mol Endocrinol 2001;15:501-11.
29. Lemée F, Bavoux C, Pillaire MJ, et al. Characterization of promoter regulatory elements involved in downexpression of the DNA polymerase kappa in colorectal cancer. Oncogene 2007;26:3387-94.
30. Rácz GA, Nagy N, Tóvári J, Apáti Á, Vértessy BG. Identification of new reference genes with stable expression patterns for gene expression studies using human cancer and normal cell lines. Sci Rep 2021;11:19459.
31. Marisa L, de Reyniès A, Duval A, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med 2013;10:e1001453.
32. Roepman P, Schlicker A, Tabernero J, et al. Colorectal cancer intrinsic subtypes predict chemotherapy benefit, deficient mismatch repair and epithelial-to-mesenchymal transition. Int J Cancer 2014;134:552-62.
33. Cortazar AR, Torrano V, Martín-Martín N, et al. CANCERTOOL: a visualization and representation interface to exploit cancer datasets. Cancer Res 2018;78:6320-8.
34. Salazar R, Roepman P, Capella G, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol 2011;29:17-24.
35. Pillaire MJ, Selves J, Gordien K, et al. A ‘DNA replication’ signature of progression and negative outcome in colorectal cancer. Oncogene 2010;29:876-87.
36. Nagy Á, Munkácsy G, Győrffy B. Pancancer survival analysis of cancer hallmark genes. Sci Rep 2021;11:6047.
37. Györffy B, Lanczky A, Eklund AC, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 2010;123:725-31.
38. Augereau P, Badia E, Balaguer P, et al. Negative regulation of hormone signaling by RIP140. J Steroid Biochem Mol Biol 2006;102:51-9.
39. Castet A, Herledan A, Bonnet S, et al. Receptor-interacting protein 140 differentially regulates estrogen receptor-related receptor transactivation depending on target genes. Mol Endocrinol 2006;20:1035-47.
40. Wang Y, Seimiya M, Kawamura K, et al. Elevated expression of DNA polymerase kappa in human lung cancer is associated with p53 inactivation: Negative regulation of POLK promoter activity by p53. Int J Oncol 2004;25:161-5.
41. Wit N, Buoninfante OA, van den Berk PC, et al. Roles of PCNA ubiquitination and TLS polymerases κ and η in the bypass of methyl methanesulfonate-induced DNA damage. Nucleic Acids Res 2015;43:282-94.
42. Ogi T, Mimura J, Hikida M, Fujimoto H, Fujii-Kuriyama Y, Ohmori H. Expression of human and mouse genes encoding polkappa: testis-specific developmental regulation and AhR-dependent inducible transcription. Genes Cells 2001;6:943-53.
43. Madak-Erdogan Z, Katzenellenbogen BS. Aryl hydrocarbon receptor modulation of estrogen receptor α-mediated gene regulation by a multimeric chromatin complex involving the two receptors and the coregulator RIP140. Toxicol Sci 2012;125:401-11.
44. Wei LN, Hu X, Chandra D, Seto E, Farooqui M. Receptor-interacting protein 140 directly recruits histone deacetylases for gene silencing. J Biol Chem 2000;275:40782-7.
45. Friedberg EC. Suffering in silence: the tolerance of DNA damage. Nat Rev Mol Cell Biol 2005;6:943-53.
46. Bétous R, Pillaire MJ, Pierini L, et al. DNA polymerase κ-dependent DNA synthesis at stalled replication forks is important for CHK1 activation. EMBO J 2013;32:2172-85.
47. Wang H, Wu W, Wang HW, et al. Analysis of specialized DNA polymerases expression in human gliomas: association with prognostic significance. Neuro Oncol 2010;12:679-86.
48. Bavoux C, Leopoldino AM, Bergoglio V, et al. Up-regulation of the error-prone DNA polymerase {kappa} promotes pleiotropic genetic alterations and tumorigenesis. Cancer Res 2005;65:325-30.
49. Allera-Moreau C, Rouquette I, Lepage B, et al. DNA replication stress response involving PLK1, CDC6, POLQ, RAD51 and CLASPIN upregulation prognoses the outcome of early/mid-stage non-small cell lung cancer patients. Oncogenesis 2012;1:e30.
50. Pan Q, Fang Y, Xu Y, Zhang K, Hu X. Down-regulation of DNA polymerases kappa, eta, iota, and zeta in human lung, stomach, and colorectal cancers. Cancer Lett 2005;217:139-47.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Palassin P, Lapierre M, Bonnet S, Pillaire MJ, Győrffy B, Teyssier C, Jalaguier S, Hoffmann JS, Cavaillès V, Castet-Nicolas A. RIP140 regulates POLK gene expression and the response to alkylating drugs in colon cancer cells . Cancer Drug Resist 2022;5:401-14. http://dx.doi.org/10.20517/cdr.2021.133
AMA Style
Palassin P, Lapierre M, Bonnet S, Pillaire MJ, Győrffy B, Teyssier C, Jalaguier S, Hoffmann JS, Cavaillès V, Castet-Nicolas A. RIP140 regulates POLK gene expression and the response to alkylating drugs in colon cancer cells . Cancer Drug Resistance. 2022; 5(2): 401-14. http://dx.doi.org/10.20517/cdr.2021.133
Chicago/Turabian Style
Palassin, Pascale, Marion Lapierre, Sandrine Bonnet, Marie-Jeanne Pillaire, Balázs Győrffy, Catherine Teyssier, Stéphan Jalaguier, Jean-Sébastien Hoffmann, Vincent Cavaillès, Audrey Castet-Nicolas. 2022. "RIP140 regulates POLK gene expression and the response to alkylating drugs in colon cancer cells " Cancer Drug Resistance. 5, no.2: 401-14. http://dx.doi.org/10.20517/cdr.2021.133
ACS Style
Palassin, P.; Lapierre M.; Bonnet S.; Pillaire M.J.; Győrffy B.; Teyssier C.; Jalaguier S.; Hoffmann J.S.; Cavaillès V.; Castet-Nicolas A. RIP140 regulates POLK gene expression and the response to alkylating drugs in colon cancer cells . Cancer Drug Resist. 2022, 5, 401-14. http://dx.doi.org/10.20517/cdr.2021.133
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 8 clicks
Cite This Article 8 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.