Liquid biopsy: expanding the frontier of circulating biomarker discovery and validation in breast cancer
Abstract
Liquid biopsies represent an attractive, minimally-invasive alternative to surgical sampling or complex imaging of breast cancer and breast cancer metastasis. Here we present a summary of the major biomarker components often evaluated in liquid biopsy samples from patients with breast cancer, including circulating tumor cells, circulating cell-free tumor DNA, and cancer-associated plasma proteins. We discuss recent advancements in methods of detection and use of these biomarkers in breast cancer. Finally, we highlight some of our own recent contributions to breast cancer liquid biopsy, including the identification and characterization of circulating Cancer Associated Fibroblasts.
Keywords
Introduction
Since the first reported observation of circulating tumor cells (CTCs) by Ashworth[1] in 1869, there has been significant interest in assessing CTCs and other circulating biomarkers in cancer patients, their associations with disease state and prognosis, and their potential value as predictors of treatment response or tools to monitor disease progression. The idea of a “liquid biopsy” that enables the assessment of informative biomarkers through minimally invasive means is a concept that is widely supported among patients, clinicians, and researchers. Currently, the biomarkers most commonly evaluated in liquid biopsy studies are CTCs, circulating cell-free tumor DNA (ctDNA), and protein biomarkers found in plasma or serum. In the last decade, many competing and complementary technologies and methods have been developed for the detection of cancer associated markers in circulation. However, it remains a challenge to identify the best way to use these markers as tools to help guide breast cancer therapy. A major challenge in the treatment of breast cancer is the emergence of therapeutic resistance. The ability to monitor breast cancer responses to therapy in real-time to determine the need for alternative treatment in the event that a tumor develops resistance would revolutionize breast cancer care. Currently, studies on long-term treatment efficacy are limited by the need to monitor actual recurrent breast cancer. Liquid biopsy boasts the potential to follow one or more circulating biomarkers in real time as a surrogate for therapeutic success or failure. Our recent collaborative research efforts have focused on the discovery and validation of circulating cancer associated fibroblasts (cCAFs) as a novel cancer biomarker, and the use of liquid biopsy to monitor levels of cCAFs and other circulating biomarkers associated with breast cancer stage, metastasis, survival, and therapeutic response.
Circulating cell-free tumor DNA is a prognostic and predictive marker of breast cancer
Recent developments in genomic technology enable the large-scale use of massively parallel DNA sequencing to detect genomic alterations in tumor samples. Targeted mutation panels (such as the FoundationOne® Liquid or Guardant360® tests) identify mutations in commonly mutated cancer-driver genes, which can be used to monitor treatment response or progression of many tumor types. ctDNA consists of small fragments of tumor DNA found in circulation that are not contained within cells[2]. ctDNA is used as a biomarker to identify specific mutations identified within a tumor that can guide treatment, and can also be used to monitor tumor progression[3,4]. Moreover, upwards of 70% of early-stage breast cancer patients have tumor-specific mutations present in ctDNA[5]. Genetic alterations present in a patient’s primary tumor biopsy can be detected in ctDNA in peripheral blood using sensitive mutation-targeted digital PCR methods[6]. Several studies have reported that detection of ctDNA in early stage breast cancer is associated with early recurrence, but further studies are needed to evaluate the use of ctDNA as an indicator of therapeutic response[5,7,8]. Recently, the Signatera™ personalized ctDNA test was shown to detect molecular indications of disease recurrence in breast cancer patients following adjuvant chemotherapy up to two years in advance of clinically detected metastatic relapse, with a sensitivity of 89% and a specificity of 100%[9]. Highlighting the potential impact of monitoring ctDNA for indications of disease recurrence, the Signatera™ test recently received a Breakthrough Device Designation from the FDA for use in detecting residual disease in patients with breast cancer, bladder cancer, non-small cell lung cancer, and colorectal cancer up to two years prior to conventional imaging methods. Furthermore, a method of personalized ctDNA detection, called “TARDIS”, which utilizes a novel targeted sequencing approach to evaluate tumor-specific mutations in ctDNA, was recently demonstrated to detect specific mutations in ctDNA as rare as 3 in 105[10]. McDonald et al.[10] used the TARDIS method to monitored patient-specific mutations in ctDNA in a breast cancer patient cohort throughout neoadjuvant treatment. This study demonstrated that breast cancer patients who achieved a pathological complete response exhibited significantly larger reductions in ctDNA, and had significantly lower ctDNA fractions, compared to patients with residual disease after neoadjuvant treatment. These studies demonstrate the utility of modern sequencing and PCR techniques to detect ctDNA as a molecular indicator of breast cancer, and indicate the potential that such methods have in monitoring therapeutic response and disease progression in breast cancer.
Circulating tumor cells as prognostic biomarkers in breast cancer
In order for breast cancer to metastasize, breast cancer cells must escape the primary tumor site, enter into and travel through the circulation, exit the vasculature and invade into distant organ sites, and establish distant secondary lesions. CTCs are cancer cells that are found in circulation, and they provide predictive and prognostic information about a patient’s breast cancer. CTCs have been shown to have prognostic value in both metastatic and early breast cancer. In the context of metastatic breast cancer, high CTC counts are associated with poorer disease prognosis, and studies have shown that ~44% of patients with metastatic TNBC have ≥ 5 CTCs per 7.5 mL of blood, based on CellSearch®, the only currently FDA approved CTC enumeration platform[11]. In the context of early breast cancer, the SUCCESS trial demonstrated that the presence of CTCs at baseline prior to adjuvant chemotherapy, as well as the persistence of CTCs following adjuvant chemotherapy, was significantly associated with poorer disease free survival and worse overall survival in patients with early breast cancer[12]. A prospective study by Lucci et al.[13] demonstrated that the detection of at least one CTC was prognostic of poorer progression-free survival among chemonaive patients with early stage breast cancer. Additional studies have demonstrated that the sustained presence of CTCs following neoadjuvant chemotherapy is associated with resistance to therapy[14], and that persistent detection of CTCs in breast cancer patients following adjuvant therapy predicts poorer disease-free survival (DFS) and overall survival (OS)[15]. Furthermore, DFS and OS outcomes in patients whose CTCs disappeared after therapy were comparable to outcomes in patients lacking CTCs at baseline analysis[15]. These data indicate that changes in CTCs may be prognostic for patients with breast cancer, and suggest that interventions causing a durable reduction in CTCs lead to more favorable clinical outcomes for breast cancer patients. However, it remains unclear if reductions in the number of CTCs in response to treatment directly indicate a favorable response to a specific therapeutic regimen[16,17].
Of great interest is the potential for CTCs to be used to guide intervention strategies and disease management for breast cancer. CTCs have been explored as biomarkers indicative of late breast cancer relapse. A study by Sparano et al.[18] demonstrated that detection of CTC events five years after initial diagnosis was associated with a 13.1-fold higher risk of disease recurrence in patients with HER2-negative hormone receptor-positive breast cancer. A large retrospective pooled analysis of patients with metastatic breast cancer recently reported that the threshold of 5 CTCs per 7.5 mL blood is clinically relevant in stratifying patients with Stage IV disease as “Stage IVindolent” and “Stage IVaggressive”, where patients with Stage IVindolent disease exhibited significantly longer overall survival times, regardless of breast cancer subtype, disease location, or prior treatment[19]. Recent data reported from the STIC CTC trial (a phase 3 non-inferiority trial wherein patients with metastatic ER+HER2- disease received hormone therapy or chemotherapy as a priori treatment choice, or were assigned to hormone therapy or chemotherapy based on CellSearch® CTC counts) indicate that not only was the treatment decision based on CTC count not inferior to the a priori treatment arm, but patients who were switched from hormone therapy to chemotherapy based on high CTC count had significantly longer progression free survival[20]. The STIC CTC trial is providing some of the first indications that CTC count can guide clinical action to not only assist with disease staging, but to improve outcomes for patients with metastatic breast cancer.
Circulating proteins in plasma or serum are indicative of breast cancer progression and metastasis
Plasma proteins derived from both breast cancer cells and from the tumor microenvironment can be informative biomarkers of breast cancer progression and metastasis. Levels of carcinoembryonic antigen (CEA) and cancer antigen 15-5 (CA 15-3, MUC1) are independent prognostic markers for poor disease-free survival outcomes in patients with TNBC[21]. In patients with metastatic breast cancer, greater reductions in CA 15-3 levels during first-line chemotherapy significantly associated with improved time-to-progression and improved survival[22]. Furthermore, longitudinal increases in CA 15-3 and CEA may also provide early indications of breast cancer metastasis[23,24]. Cancer antigen 125 (CA 125) has been found to associate with the presence of pleural breast cancer metastasis[25]. Inclusion of CA 125 in a protein biomarker panel along with CA 15-3 demonstrated improved sensitivity to detect early breast cancer recurrence[26]. The utility of these proteins in the detection, response, and monitoring of breast cancer is achievable through minimally-invasive liquid biopsies, and can be performed in synchronization with assessment of other circulating biomarkers, such as ctDNA or CTCs.
Non-epithelial CTCs, CTC clusters, and circulating cancer-associated cells
In breast cancer, CTCs have been defined in a variety of ways, most commonly based on the presence of EpCAM[27] or epithelial cytokeratins[28], and the absence of CD45[27,28]. However, CTCs lacking classical epithelial markers and have been also reported in patients with breast cancer[29,30]. CTCs with mesenchymal characteristics, including protein expression of vimentin[31] and N-cadherin[32], have been detected, demonstrating the phenotypic heterogeneity exhibited by breast cancer CTCs. Detection of CTCs expressing cancer stemness factors and undergoing epithelial-to-mesenchymal transition has been shown to indicate chemoresistance and poor clinical outcome in patients with metastatic breast cancer[33]. The emergence of breast cancer CTCs that do not fit within the widely accepted EpCAM-positive or epithelial cytokeratin-positive definitions demonstrates the importance of considering broader definitions of CTCs for better prognostic and predictive application of CTC enumeration.
The presence of CTCs in clusters with other CTCs (“homotypic clusters”) has also been reported, and is thought to indicate the viability and metastatic potential of CTCs. Preclinical models of cancer metastasis have demonstrated that CTCs in clusters, rather than individual CTCs, are more readily able to establish metastasis[34,35]. Additionally, the presence CTC clusters in patients with advanced stage breast cancer has been shown to be prognostic of poor outcome[36-38]. Exploration into the mechanisms of CTC clustering has shown that aggregation of CTCs in circulation occurs by homophilic CD44-CD44 interactions, and associates with advanced disease in preclinical mouse models. Furthermore, the presence of CD44+ CTC clusters in patients with metastatic breast cancer was found to significantly associate with poorer overall survival[38,39].
In addition to circulating cancer cells, other circulating cancer-associated cells have been reported, including neutrophils[40], macrophages[41], and cancer-associated giant macrophage-like cells[42]. Preclinical models of breast cancer metastasis have shown that cells from the primary tumor stroma, including cancer associated fibroblasts (CAFs) can accompany breast cancer cells in circulation, ultimately facilitating metastatic dissemination[43]. These cells may circulate independently or in clusters with CTCs, and the significance of these circulating cancer-associated cells to breast cancer metastasis and the effects that they may have on treatment efficacy are of great interest.
Cancer associated fibroblasts as a circulating biomarker in breast cancer
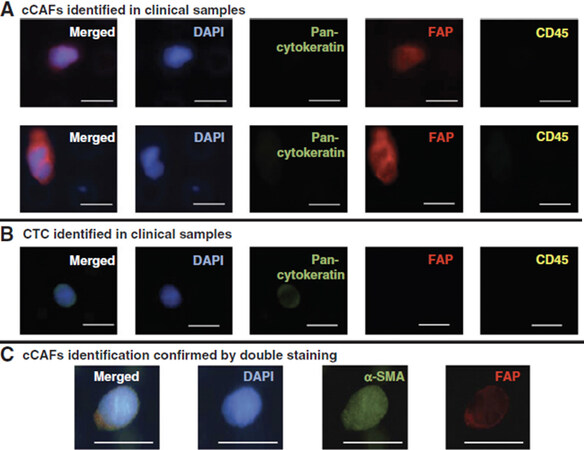
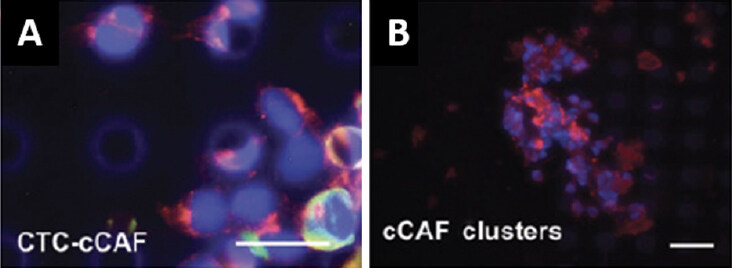
Our initial forays into liquid biopsy in breast cancer focused on the capture and enumeration of CTCs and the evaluation of serum and plasma protein biomarkers. In collaboration with Drs. Ram Datar and Richard Cote, we used a size-based microfiltration platform to capture circulating cells from liquid biopsy samples, and we identified those cells based on well-established markers using immunofluorescent staining. In addition to circulating breast cancer cells, we observed other cells that were negative for cytokeratin and negative for CD45. Prior observations by Duda et al.[43] that CTCs can carry elements of the tumor microenvironment, including fibroblasts[43], and reports by others of fibroblast-like cells in circulation of cancer patients[44,45], suggested to us that these cells may be cancer associated fibroblasts. We found that many of these cells of indeterminate identity were positive for FAP and α-SMA (widely accepted markers for CAFs), and lacked cytokeratin expression or expression of the leukocyte common antigen, CD45, and we described this population of cells as circulating CAFs (cCAFs) [Figure 1]. Additionally, when isolated and put into culture, these cells exhibited characteristic CAF morphology. We reported that cCAFs are absent from healthy individuals, and are rarely seen in patients considered “cured” of breast cancer; i.e., minimum 5-years no evidence of disease. Of extreme interest to us, we discovered CTCs in circulating clusters with these cCAFs, as well as clusters containing only cCAFs [Figure 2], suggesting to us that these cCAFs are more than a putative cancer biomarker, and may be involved in mediating the metastatic process[46]. In an expanded cohort of breast cancer patients, we discovered that cCAFs and clusters of cCAFs with CTCs, which we describe as “heterotypic cCAF-CTC clusters” can be found in patients with earlier stage breast cancer (Sharma et al., unpublished data, manuscriopt pending review). The significance of these heterotypic cCAF-CTC clusters is not yet known, but we speculate may be informative for occult disseminated disease in early-stage breast cancer, and may serve as a novel prognostic biomarker.
Figure 1. Identification of circulating cancer associated fibroblasts (cCAFs) in whole blood liquid biopsy samples from patients with metastatic breast cancer. A: cCAFs identified in clinical samples; B: circulating tumor cells (CTCs) identified in clinical samples; C: cCAFs identification confirmed by double staining. Scale bar = 20 µm. Data reproduced from Ao et al.[46]
Figure 2. Clusters of circulating cells detected in whole blood liquid biopsy samples from a patient with metastatic breast cancer. A: representative heterotypic cluster of circulating tumor cells (CTCs) and circulating cancer associated fibroblasts (cCAFs); B: representative homotypic cluster of cCAFs. Blue: DAPI; Red: FAP; Green: Cytokeratin; White: CD45. Scale bar = 20 µm. Data reproduced from Ao et al.[46]
To further evaluate the potential role of cCAFs in breast cancer metastasis, we developed a co-injection xenograft model, wherein human breast cancer cells are co-implanted with our primary human CAF cells. Consistent with previous reports, we observed not only increased primary tumor growth induced by co-injection of CAFs and cancer cells[47,48], but increased disease metastasis (Sharma et al., unpublished data, manuscriopt pending review). In these xenograft models, we found human CAFs in circulation and present at sites of metastasis, confirming our notion that cCAFs represent CAFs from primary breast tumors that have entered the circulation. Additionally, we also observed clusters of circulating cells; these clusters often consisted of CTCs, cCAFs, or both CTCs and cCAFs, consistent with our observations from breast cancer patients. We further recapitulated these observations in syngeneic and spontaneous murine models of breast cancer metastasis. In addition, we observed cCAF/CTC heterotypic clusters far more often in models of metastatic breast cancer, compared to non-metastatic or poorly metastatic models. Our studies in these models show that the presence of cCAF/CTC heterotypic clusters is likely related to a breast cancer’s metastatic propensity.
Goals and future directions of liquid biopsy in breast cancer
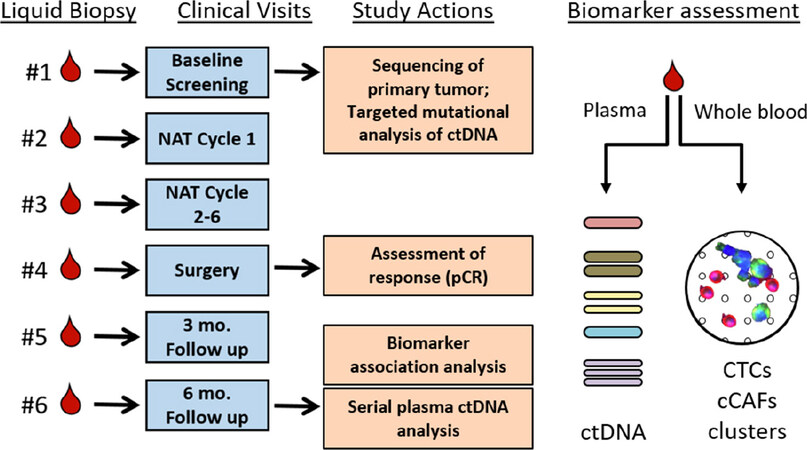
Our immediate goals are to advance the use of liquid biopsy to assess therapeutic responses in primary breast cancer and to monitor disease recurrence in the post-treatment setting. Moreover, a liquid biopsy approach that assesses genomic, cellular, and protein biomarkers could be expanded to sequence individual CTCs to correlate ctDNA data with clonal data directly obtained from CTC populations as well as sequence CTCs within heterotypic clusters. The compelling demonstration that assessment of circulating factors provides predictive and prognostic information of treatment efficacy would change the landscape of neoadjuvant and adjuvant therapy for breast cancer patients in a fundamental way: by identifying patients who are adequately treated and those who are not. There is an impetus to explore the use of real-time monitoring of circulating biomarkers to inform changes in therapeutic approach, to provide better care and identify additional treatment options for patients who may not initially respond to therapy. Our own observations and the rapid rise of ctDNA as an informative biomarker have led us to initiate a series of prospective liquid biopsy clinical studies in breast cancer patients to determine if longitudinal changes in circulating cellular biomarkers (CTCs, cCAFs, CTC homo/heterotypic clusters) and circulating cell-free tumor DNA are informative biomarkers of breast cancer response or resistance to neoadjuvant and adjuvant therapy (Study schematic, Figure 3). In these prospective studies, we will also collect plasma samples, enabling us to assess circulating protein markers or novel circulating DNA markers, to interrogate the utility of a more comprehensive liquid biopsy incorporating circulating cellular, protein, and genetic elements as a means of monitoring breast cancer treatment response in a minimally invasive manner. Our studies will complement other concurrent ongoing clinical trials to demonstrate the utility of liquid biopsy in monitoring disease progression and in therapeutic decision-making for breast cancer patients.
Figure 3. Schematic of study to simultaneously evaluate mutations in circulating cell-free tumor DNA (ctDNA), circulating tumor cells (CTCs), circulating cancer associated fibroblasts (cCAFs), and clusters of circulating cells in breast cancer patients undergoing neoadjuvant chemotherapy. Longitudinal assessment will determine if changes in these biomarkers associates with pathological complete response (pCR)
The potential for liquid biopsy methods to provide a minimally invasive means to detect breast cancer, predict treatment efficacy, and monitor disease recurrence is emphasized by recent efforts to encourage innovation and career training in the liquid biopsy space. New research is constantly improving on methods leading to improved sensitivity and specificity for circulating biomarkers in breast cancer detection. Novel biomarkers, including circulating microRNAs, novel populations of circulating cells, and circulating methylated DNA are being explored to compete with or complement existing circulating biomarkers to improve the utility of liquid biopsy for breast cancer care. Finally, the development of tumor-specific biomarker panels is advancing the use of liquid biopsy in personalized precision medicine. Liquid biopsy continues to represent a broad frontier for scientific discovery and a path of hope for patients with breast cancer and those at risk of developing breast cancer.
Declarations
AcknowledgmentsThe work described here represent many years of collaboration and continued study, and would not have been possible without the following contributions: Utsav Sharma, Kelsie Medina-Saenz, Angela Sparz, Benjamin Tronnes, Leah Machlin, and Sanket Shah for developing and refining methodology for clinical and preclinical study of cCAFs and heterotypic clusters; Ana Sandoval and Susan Kesmodel for clinical study activities at the University of Miami; Zheng Ao, Anthony Williams, Siddarth Rawal, Ram Datar, and Richard Cote, for development of the microfilter platform.
Authors’ contributionsMade substantial contribution to the content, composition, and discussion reported here, designed and implemented the preclinical studies and clinical trials referenced here: Miller PC, El-Ashry D, Lippman ME
Availability of data and materialsNot applicable.
Financial support and sponsorshipOur work referenced here was supported by funding from the Breast Cancer Research Foundation (BCRF16098), and by the Prevent Cancer Foundation (M1601095).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateOur clinical study described here was approved by the Institutional Review Board of the University of Miami. All patients involved provided written informed consent.
Consent for publicationNot applicable.
Copyright© The Author(s) 2019.
REFERENCES
1. Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J 1869;14:146.
2. Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer--a survey. Biochim Biophys Acta 2007;1775:181-232.
3. Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014;6:224ra24.
4. Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013;368:1199-209.
5. Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015;7:302ra133.
6. De Mattos-Arruda L, Caldas C. Cell-free circulating tumour DNA as a liquid biopsy in breast cancer. Mol Oncol 2016;10:464-74.
7. Olsson E, Winter C, George A, Chen Y, Howlin J, et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med 2015;7:1034-47.
8. Reinert T, Scholer LV, Thomsen R, Tobiasen H, Vang S, et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut 2016;65:625-34.
9. Coombes RC, Page K, Salari R, Hastings RK, Armstrong A, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res 2019;25:4255-63.
10. McDonald BR, Contente-Cuomo T, Sammut SJ, Odenheimer-Bergman A, Ernst B, et al. Personalized circulating tumor DNA analysis to detect residual disease after neoadjuvant therapy in breast cancer. Sci Transl Med 2019;11:eaax7392.
11. Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014;15:406-14.
12. Rack B, Schindlbeck C, Juckstock J, Andergassen U, Hepp P, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 2014;106.
13. Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 2012;13:688-95.
14. Smerage JB, Hayes DF, Doyle GV, Terstappen LW, Brown ME, et al. Assessment of circulating tumor cells in breast cancer patients undergoing neoadjuvant chemotherapy. J Clin Oncol 2006; doi: 10.1200/jco.2006.24.18_suppl.10079.
15. Saloustros E, Perraki M, Apostolaki S, Kallergi G, Xyrafas A, et al. Cytokeratin-19 mRNA-positive circulating tumor cells during follow-up of patients with operable breast cancer: prognostic relevance for late relapse. Breast Cancer Res 2011;13:R60.
16. Pachmann K, Camara O, Kavallaris A, Schneider U, Schunemann S, et al. Quantification of the response of circulating epithelial cells to neodadjuvant treatment for breast cancer: a new tool for therapy monitoring. Breast Cancer Res 2005;7:R975-9.
17. Riethdorf S, Muller V, Zhang L, Rau T, Loibl S, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 2010;16:2634-45.
18. Sparano J, O’Neill A, Alpaugh K, Wolff AC, Northfelt DW, et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 2018;4:1700-6.
19. Cristofanilli M, Pierga JY, Reuben J, Rademaker A, Davis AA, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit Rev Oncol Hematol 2019;134:39-45.
20. Bidard FC, Jacot W, Dureau S, Brain E, Bachelot T, et al. Abstract GS3-07: clinical utility of circulating tumor cell count as a tool to chose between first line hormone therapy and chemotherapy for ER+ HER2- metastatic breast cancer: results of the phase III STIC CTC trial. Cancer Res 2019;79:GS3-07.
21. Dai D, Chen B, Tang H, Wang B, Zhao Z, et al. Nomograms for predicting the prognostic value of pre-therapeutic CA15-3 and CEA serum levels in TNBC patients. PLoS One 2016;11:e0161902.
22. Tampellini M, Berruti A, Bitossi R, Gorzegno G, Alabiso I, et al. Prognostic significance of changes in CA 15-3 serum levels during chemotherapy in metastatic breast cancer patients. Breast Cancer Res Treat 2006;98:241-8.
23. Pons-Anicet DM, Krebs BP, Mira R, Namer M. Value of CA 15:3 in the follow-up of breast cancer patients. Br J Cancer 1987;55:567-9.
24. Saw S, Lim J, Lim SH, Wong M, Lim C, et al. Patterns of relapse after neoadjuvant chemotherapy in breast cancer: implications for surveillance in clinical practice. Breast Cancer Res Treat 2019;177:197-206.
25. Kramer S, Jager W, Lang N. CA 125 is an indicator for pleural metastases in breast cancer. Anticancer Res 1997;17:2967-70.
26. Di Gioia D, Blankenburg I, Nagel D, Heinemann V, Stieber P. Tumor markers in the early detection of tumor recurrence in breast cancer patients: CA 125, CYFRA 21-1, HER2 shed antigen, LDH and CRP in combination with CEA and CA 15-3. Clin Chim Acta 2016;461:1-7.
27. Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21.
28. Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23:1420-30.
29. Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013;339:580-4.
30. Konigsberg R, Obermayr E, Bises G, Pfeiler G, Gneist M, et al. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol 2011;50:700-10.
31. Satelli A, Brownlee Z, Mitra A, Meng QH, Li S. Circulating tumor cell enumeration with a combination of epithelial cell adhesion molecule- and cell-surface vimentin-based methods for monitoring breast cancer therapeutic response. Clin Chem 2015;61:259-66.
32. Kaigorodova EV, Savelieva OE, Tashireva LA, Tarabanovskaya NA, Simolina EI, et al. Heterogeneity of circulating tumor cells in neoadjuvant chemotherapy of breast cancer. Molecules 2018;23.
33. Papadaki MA, Stoupis G, Theodoropoulos PA, Mavroudis D, Georgoulias V, et al. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol Cancer Ther 2019;18:437-47.
34. Esposito A, Bardelli A, Criscitiello C, Colombo N, Gelao L, et al. Monitoring tumor-derived cell-free DNA in patients with solid tumors: clinical perspectives and research opportunities. Cancer Treat Rev 2014;40:648-55.
35. Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014;158:1110-22.
36. Jansson S, Bendahl PO, Larsson AM, Aaltonen KE, Ryden L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 2016;16:433.
37. Mu Z, Benali-Furet N, Uzan G, Znaty A, Ye Z, et al. Detection and characterization of circulating tumor associated cells in metastatic breast cancer. Int J Mol Sci 2016;17.
38. Wang C, Mu Z, Chervoneva I, Austin L, Ye Z, et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat 2017;161:83-94.
39. Wang J, Cao MG, You CZ, Wang CL, Liu SL, et al. A preliminary investigation of the relationship between circulating tumor cells and cancer stem cells in patients with breast cancer. Cell Mol Biol (Noisy-le-grand) 2012;58 Suppl:OL1641-5.
40. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019;566:553-7.
41. Zhang B, Cao M, He Y, Liu Y, Zhang G, et al. Increased circulating M2-like monocytes in patients with breast cancer. Tumour Biol 2017;39:1010428317711571.
42. Adams DL, Martin SS, Alpaugh RK, Charpentier M, Tsai S, et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc Natl Acad Sci U S A 2014;111:3514-9.
43. Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A 2010;107:21677-82.
44. Ishii G, Ito TK, Aoyagi K, Fujimoto H, Chiba H, et al. Presence of human circulating progenitor cells for cancer stromal fibroblasts in the blood of lung cancer patients. Stem Cells 2007;25:1469-77.
45. Jones ML, Siddiqui J, Pienta KJ, Getzenberg RH. Circulating fibroblast-like cells in men with metastatic prostate cancer. Prostate 2013;73:176-81.
46. Ao Z, Shah SH, Machlin LM, Parajuli R, Miller PC, et al. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res 2015;75:4681-7.
47. Tyan SW, Kuo WH, Huang CK, Pan CC, Shew JY, et al. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PLoS One 2011;6:e15313.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Miller PC, El-Ashry D, Lippman ME. Liquid biopsy: expanding the frontier of circulating biomarker discovery and validation in breast cancer. Cancer Drug Resist 2019;2:1215-23. http://dx.doi.org/10.20517/cdr.2019.99
AMA Style
Miller PC, El-Ashry D, Lippman ME. Liquid biopsy: expanding the frontier of circulating biomarker discovery and validation in breast cancer. Cancer Drug Resistance. 2019; 2(4): 1215-23. http://dx.doi.org/10.20517/cdr.2019.99
Chicago/Turabian Style
Miller, Philip C., Dorraya El-Ashry, Marc E. Lippman. 2019. "Liquid biopsy: expanding the frontier of circulating biomarker discovery and validation in breast cancer" Cancer Drug Resistance. 2, no.4: 1215-23. http://dx.doi.org/10.20517/cdr.2019.99
ACS Style
Miller, PC.; El-Ashry D.; Lippman ME. Liquid biopsy: expanding the frontier of circulating biomarker discovery and validation in breast cancer. Cancer Drug Resist. 2019, 2, 1215-23. http://dx.doi.org/10.20517/cdr.2019.99
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 9 clicks
Cite This Article 9 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.