Resistance mechanism to cisplatin in NCI-H460 non-small cell lung cancer cell line: investigating apoptosis, autophagy, and cytogenetic damage
Abstract
Aim: To investigate the effects of cisplatin on the human non-small cell lung carcinoma (NCI-H460) cell line regarding cytotoxicity, genotoxicity, and expression of genes associated with apoptosis (BIRC5) and autophagy (BECN1).
Methods: Cell cultures were treated with cisplatin concentrations (0.16-33.3 μmol/L) for 48 h. Mutagenicity and acute and chronic cytotoxicities were assessed using the MTT, clonogenic, and cytokinesis-block micronucleus assays. Gene expression of BIRC5 and BECN1 was evaluated by reverse transcription-polymerase chain reaction.
Results: Cisplatin IC50 (0.33 μmol/L) increased micronucleus frequency 2.50 times. Cisplatin was also cytotoxic in the 0.6-33.3 μmol/L range, with reduced expression of the BIRC5 gene, suggesting induction of apoptosis. Besides reducing the expression of the BIRC5 gene, 33.3 μmol/L cisplatin increased the expression of the BECN1 gene, suggesting that autophagy can be related to cisplatin resistance.
Conclusion: Cisplatin inhibited NCI-H460 growth, and cisplatin IC50 induced genotoxic damage. When higher cisplatin concentrations are used, the expression of genes associated with apoptosis and autophagy was changed. This results points to a further investigation of the role of autophagy in cisplatin resistance.
Keywords
Introduction
Lung cancer is the most common cause of death from cancer worldwide, accounting for 1.69 million deaths in 2015[1]. As an aggressive tumor with poor prognosis and a cumulative mean total survival of over 5 years, it is most prevalent in men between 65-70 years of age and chronic tobacco users[2,3]. Lung cancer is divided into two types: small cell lung carcinoma and non-small cell lung carcinoma (NSCLC). The latter accounts for 85% to 90% of all lung cancer cases[4,5].
The choice of treatment for NSCLC has to consider a number of factors, such as tumor type, staging, and the overall clinical condition of the patient. For more than two decades, the most effective systemic chemotherapy for NSCLC included cisplatin or other platinum-based combinations, which remains the standard first-line chemotherapy for advanced NSCLC until today[6,7]. Cisplatin monotherapy in patients with advanced NSCLC is usually given at a dosage of 50-120 mg.m-2 per cycle[8].
Cisplatin binds to DNA, forming adducts that will turn into intra- and inter-chain cross-links, twisting the DNA helix, inhibiting replication, interfering with DNA repair mechanisms, and directing cells to apoptosis[9]. Cisplatin also inhibits RNA transcription and induced G2 cell cycle arrest, and/or apoptosis. Previous studies have shown that platinum complexes may be carcinogenic and mutagenic. They are able to induce chromosomal aberrations and increase micronucleus (MN) frequency in human lymphocytes and mice and rat bone marrow cell cultures. Importantly, it is believed that genotoxicity is an important mechanism in the development of cisplatin toxicity[10,11].
Despite the efficacy of this antineoplastic drug in NSCLC treatment protocols, the clinical uses of cisplatin are limited due to its toxic effect and development of cellular resistance, which significantly restricts the tolerable range of therapeutic doses[12,13]. In this sense, low sensitivity or even chemoresistance indicate the risk of poor prognosis, increasing the economic burden to patients. Resistance to cisplatin is frequently reported in cancer patients[14], and may be caused by overexpression of multidrug resistance transport genes, activation of DNA repair mechanism, glutathione conjugation, defects in cell cycle arrest, and apoptosis[15]. More recently it was postulated that cisplatin induces autophagy as a resistance mechanism for cancer cell survival[16].
Considering that platinum complexes still have potentially interesting chemotherapeutic properties, efforts to understand the mechanisms of both resistance and sensitivity to cisplatin are of great importance in NSCLC treatment. Thus, the objective of this study was to evaluate cytotoxicity, genotoxicity, and expression of genes associated with apoptosis (BIRC5) and autophagy (BECN1) in response to treatment with cisplatin in human NSCLC NCI-H460 cell line.
Methods
Cell line and cultures maintenance
The human NSCLC NCI-H460 cell line was purchased from the American Type Culture Collection (Rockville, MD, USA). Cells were maintained in 25-cm2 or 75-cm2 culture flasks with Eagle modified Dulbecco’s culture medium (DMEM, Invitrogen Corporation, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal calf bovine serum (FBS, Cultilab, Campinas, SP, Brazil) at 37 ºC in a humid atmosphere containing 5% CO2.
Cisplatin treatment and cytotoxicity assessment
Acute cytotoxicity was evaluated using the colorimetric MTT assay [3- (4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; Life Technologies, Oregon, USA][17,18]. For this evaluation, 3 × 104 cells were inoculated in 96-well microplates and submitted to serial dilutions (0.16-33.3 μmol/L) of cisplatin (LIBBS Pharmaceuticals, São Paulo, SP, Brazil). After 48 h of exposure, the cells were incubated with 100 μL of a MTT solution in serum-free and phenol-free culture medium at 37 °C for 3 h. After, the supernatant was removed and the violet formazan crystals formed were solubilized in 100 μL DMSO. The assay was carried out in a microplate reader (Multiskan, Uniscience, São Paulo, SP, Brazil) at an optical density of 540 nm. The IC50 value was derived from the dose-response profile.
Cell colony formation assay
Cell cultures were submitted to treatment with cisplatin (0.33-33.3 μmol/L) for 48 h. Next, they were removed using trypsin-EDTA 0.10%, and 300 cells/well were seeded in 6-well plates. Incubation at 37 ºC for 7 days ensued, when the culture medium was removed, washed with phosphate buffered saline (PBS) and then ethanol 70% (2 mL/well) for 1 h at 4 ºC for colony fixation[19]. Then, ethanol was removed and plates were left to dry at room temperature. Subsequently, colonies were stained with crystal violet (1 mL/well) for 30 min at room temperature and quantified using an inverted microscope. The results were expressed as survival fraction (SF) using the formula SF = T/C ∙ 100, where T is the number of colonies after treatment and C is the number of colonies in non-treated controls.
Evaluation of genotoxicity using the cytokinesis-block micronucleus assay
Briefly, NCI-H460 cells (105 cells per well) were seeded in 24-well plates in Dulbecco’s medium supplemented with 10% FBS and incubated at 37 ºC for 24 h in 5% CO2. The cisplatin concentrations tested were determined using the MTT assay. The concentrations used were IC50 (0.33 μmol/L), half the IC50 value (0.16 μmol/L), and half this value again (0.08 μmol/L). After treatment for 48 h, cells were washed with Dulbecco’s phosphate buffered saline (DPBS). Cytochalasin B (Cyt B, Sigma-Aldrich, St. Louis, MO, USA) was added to a final concentration of 5 μg/mL in complete medium. After 28 h, Cyt B was removed and cells were washed twice with DPBS at 37 ºC, trypsinized, and gently resuspended in complete culture medium. Subsequently, cells were harvested by cytocentrifugation for 5 min at 700 rpm to produce one smear per slide. Slides were removed, fixed, and stained with Instant Prov (Newprov, Pinhais, PR, Brazil). After, slides were left to dry at room temperature and analyzed in an optical microscope (1000×). Micronuclei were scored according to Fenech[20].
Gene expression analysis
After treatment with cisplatin for 48 h, cells were stored in 1 mL of RNA stabilizing solution (RNAlater®, Ambion, Austin, TX, USA). RNA was extracted according to a standard silica-based procedure[21]. cDNA was obtained from total RNA, and gene expression was quantified using StepOnePlus™ Real-Time PCR System (Life Technologies, Carlsbad, CA, USA).
The expression of BECN1 and BIRC5 genes was evaluated using probe-based gene expression assays (Hs.PT.51.20133642 and Hs.PT.51.3536061, respectively; Integrated DNA Technologies, Coralville, IA, USA). Amplification of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was performed as internal control (Hs.PT.39a.22214836; Integrated DNA Technologies, Coralville, IA, USA). The conditions for the PCR were 95 °C for 3 min and 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Three independent experiments were performed and the algorithm 2-ΔΔCt was used to analyze the data from gene expression assays[22].
Statistical analyses
The data obtained were analyzed using Student’s t test or analysis of variance (one-way ANOVA) with Dunnett’s test as post hoc, depending on the case. All P-values are two-way and P < 0.05 indicates statistically significant differences. The statistical analyses were carried out in the Graph Prism 5.01 software (Graphpad Software Inc., La Jolla, CA, USA).
Results
Cytotoxic effect of cisplatin on NCI-H460 cell line
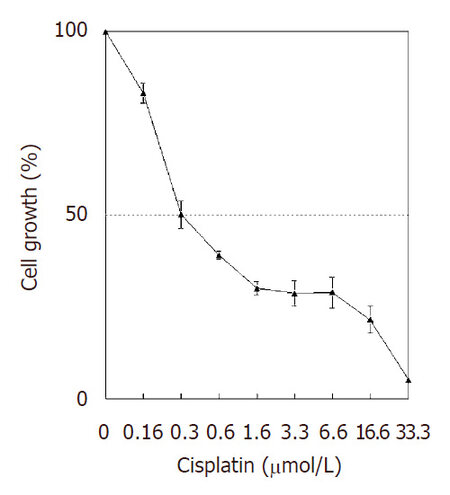
The cytotoxic effect of cisplatin on NCI-H460 cells was assessed after 48 h. All concentrations tested inhibited cell growth, with an IC50 of 0.33 ± 0.06 µmol/L [Figure 1]. Cisplatin (1.6 to 16.6 µmol/L) was highly cytotoxic (around 70% cytotoxicity); and the 33.3 µmol/L cisplatin concentration induced high antiproliferative effect (95% cytotoxicity).
Effect of cisplatin on the formation of cell colonies
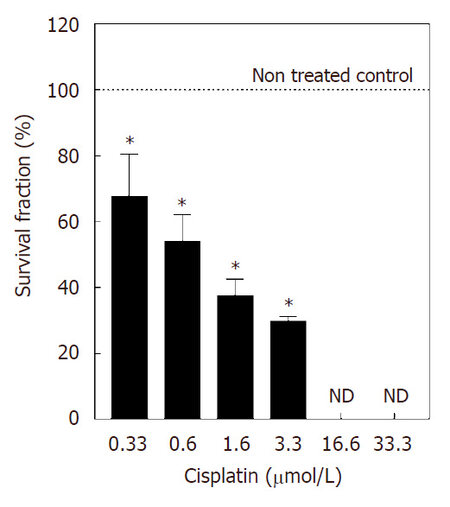
Considering that much of the cell damage induced by cisplatin may be repaired, the evaluation of the late effect of the administration of the pharmacological drug on cell proliferation should be taken into account in treatment protocols. With that in mind, the late effect of cisplatin was investigated using the clonogenic assay 7 days (without cisplatin) after treatment. Compared to the non-treated control, 0.3 μmol/L cisplatin reduced survival fraction by approximately 30%. This effect was more pronounced when the 0.6 μmol/L (50%), 1.6 μmol/L (60%), and 3.3 μmol/L (70%) concentrations were used. But cisplatin concentrations of 16.6 and 33.3 μmol/L did not allow the formation of NCI-H460 colonies [Figure 2].
Figure 2. Survival fraction of cell line NCI-H460 treated with cisplatin for 48 h. Values are expressed as mean ± standard deviation of three independent experiments with n ≥ 6. Data are presented as survival fraction obtained from the ratio of the number of treated colonies to the number of control colonies, expressed as percent values. Controls represent 100% survival fraction. *Statistically different from the control (P < 0.05). ND: not detected
Effect of cisplatin on micronucleus formation
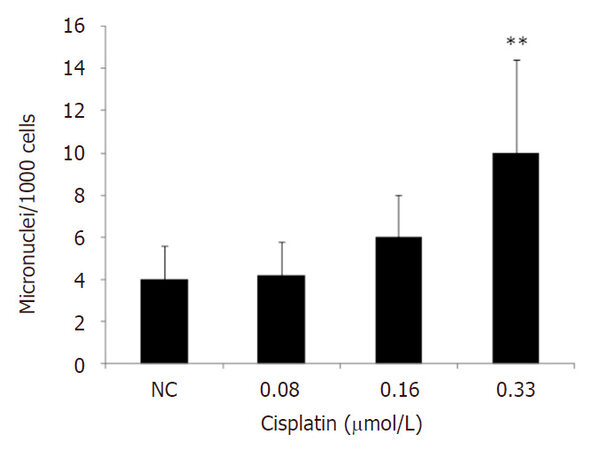
MN induction was analyzed 48 h after treatment with cisplatin. Two independent experiments were carried out in duplicate, which accounted for the analysis of 4000 binucleated cells per treatment. MN frequency increased statistically only after treatment with 0.33 μmol/L cisplatin [Figure 3].
Gene expression evaluation
Whether intrinsic or acquired, resistance to antineoplastic agents is an important clinical issue, which may lead to the failure of treatment of NSCLC. Therefore, the role of apoptosis and autophagy related genes was assessed in the response to treatment with cisplatin.
The changes in expression of the anti-apoptotic gene BIRC5, which expresses the protein survivin, indicated a dose-dependent decrease in BIRC5 expression in NCI-H460 cells (P < 0.001). For BECN1, which codifies the protein Beclin-1 and plays a role in autophagy, tumorigenesis, and neurodegeneration, expression increased only after treatment with 33.3 µmol/L cisplatin [Table 1].
Changes in gene expression of BIRC5 and BECN1 after exposure of H460 cells to different concentrations of cisplatin (0.3-33.3 µmol/L) for 48 h
| Gene | Cisplatin concentration (μmol/L) | Relative concentration |
|---|---|---|
| BIRC5 | 0.3 | 0.74 ± 0.22 |
| 0.6 | 0.30 ± 0.08** | |
| 3.3 | 0.27 ± 0.01* | |
| 6.6 | 0.21 ± 0.06** | |
| 33.3 | 0.12 ± 0.02** | |
| BECN1 | 0.3 | 1.25 ± 0.24 |
| 0.6 | 1.08 ± 0.37 | |
| 3.3 | 1.45 ± 0.08 | |
| 6.6 | 1.79 ± 0.45 | |
| 33.3 | 2.68 ± 0.60* |
Discussion
Cisplatin is commonly used to treat a wide variety of tumors. This chemotherapeutic agent is considered the treatment of choice for NSCLC, though some patients do not respond appropriately due to cellular resistance. Therefore, it is extremely important to establish the effects of cisplatin, shedding more light on the active underlying mechanisms and markers in this cell phenotype, which currently restricts the clinical use of this pharmaceutical drug in patients with lung cancer[23].
In the present study, all cisplatin concentrations (0.16-33.3 μmol/L) induced a dose-dependent antiproliferative effect in human NSCLC NCI-H460 cells. Our results confirm the findings published in a study that evaluated the cytotoxic effect of platinum complexes after 48 h of exposure of the same cell line used in our work, also using the MTT assay. The authors observed that 6 of 11 platinum complexes were significantly cytotoxic to the cell line tested, with IC50 values between 0.115 and 1.10 mmol/L[24]. Also, in non-small cell lung cancer, A459 cell line, the IC50 of cisplatin after 72 h exposure, was 9.13 µmol/L[25]. In other study using M059K glioblastoma cell line the IC50 of cisplatin was determined as 1 µmol/L[26]. The concentrations used in our study can also be compared with plasma concentration achieved after administration of cisplatin as a single agent, which ranges from 0.1-5 µmol/L[27,28].
In line with our results, it was also shown that cisplatin was not cytotoxic to NCI-H460 cells after a 24-h incubation period, though the compound induced significant inhibition of cell proliferation 48 and 72 h after treatment[29]. Similarly, no cisplatin cytotoxicity was observed after 24 h treatment of bladder carcinoma RT4, 5637, and T24 cells[30]. Also, in a human laryngeal cancer cell line Hep-2, cisplatin demonstrated cytotoxicity after 48 h exposure[31].
The percentage of cytotoxicity using MTT assay was used to indicate cell death, once when cells die, they lose the ability to convert MTT into formazan[32,33]. Based on this we can suggest that the cytotoxicity observed can indicate cell death.
The clonogenic assay was carried out to better analyze the antiproliferative effect of cisplatin on NCI-H460 cell growth. It is known that cells respond to a toxin reducing cell proliferation, when they form smaller colonies or a lower number thereof[34]. In this test, the colony-forming potential of human NSCLC NCI-H460 cells treated with 1.6 and 3.3 μmol/L cisplatin was low, suggesting that the damage caused within 48 h is permanent. The clonogenic assay determines the potential of a single cell to proliferate, when it maintains its reproductive function and may therefore form a large colony or a clone[19]. Cisplatin (0.3 and 0.6 μmol/L) also induced a significant decrease in formed colonies, endorsing previous results that indicated that cisplatin reduced clonogenic survival of strains H460 and A549 in a dose-dependent way[35]. In another study on the antiproliferative effect of cisplatin, it was observed that colony formation rate of NSCLC A459 was significantly reduced after treatment, also in a dose-dependent fashion[36]. Therefore, it is possible to conclude that the behavior of NCI-H460 cells after 7 days in culture is similar to the one observed after immediate treatment with cisplatin.
Although cisplatin has been reported to be genotoxic, studies discussing this effect on human NSCLC NCI-H460 cells are scarce. We tested the potential of cisplatin to induce MN using the IC50 and lower concentrations of the compound, since the highest concentration employed in the MN test should aim to achieve 55% ± 5% cytotoxicity[37]. The results of the present study show that 0.33 μmol/L cisplatin (IC50) increased MN frequency by 2.5 times, compared to the non-treated control group. This indicates that cisplatin causes chromosomal mutations associated with aneugenic and/or clastogenic events in human NSCLC NCI-H460 cells. Similarly, a previous study evaluated the genotoxic potential of cisplatin using the MN assay and showed that the compound and carboplatin were the most potent MN inducers (mean 13.5 and 6.0 MN/1000 binucleated cells induced by carboplatin and cisplatin, respectively)[10]. Research also shows that cisplatin induced MN formation in rat bone marrow cells[11].
Results of such relevance may be explained based on the fact that cisplatin causes DNA damage, which is measured as the presence of covalent platinum-DNA adducts. The formation of these adducts may be the preceding step to DNA cleavage during repair processes, with MN formation as a possible consequence. On the other hand, cisplatin may defunctionalize the mitotic spindle, in which case it may be considered an aneugenic compound[38,39].
BIRC5 expression regulates the mitotic spindle checkpoint and inhibits apoptosis[40]. One of the cisplatin resistance mechanisms includes the changes in regulatory pathways that control the start and progression of apoptosis. In the present study, 0.3 μmol/L cisplatin did not change BIRC5 expression significantly, suggesting that, in this concecntration, apoptosis was not induced. On the other hand, we observed a significant decrease in BIRC5 gene expression after treatment with 0.6 to 33.3 μmol/L cisplatin. This result suggests that cisplatin may inhibit BIRC5 expression, increasing the susceptibility of human NSCLC NCI-H460 cells to chemotherapy and apoptosis induction[41-43]. In this sense, it was shown, also using NCI-H460 cells, that the combination of cisplatin and arctigenin (a dibenzylbutyrolactone lignin with anti-tumor and anti-inflammatory activities) reduces survinin expression by more than 50%[41]. Recently, in lymphocytes from colon cancer patients and healthy individuals, another platinum compound, oxaliplatin, reduced surviving expression in a concentration-dependent manner, resulting in large numbers of multinucleated cells[44].
Autophagy, which is activated during metabolic stress, is a process of degradation and homeostatic cellular recycling of unnecessary or dysfunctional cytoplasmic components for energy utilization in all living cells[45,46]. Deregulation of autophagy is common among a wide range of cancers, including NSCLC, and today stands as a novel mechanism for enhancing current anticancer treatments and overcoming chemotherapy resistance[47,48]. The relationship between autophagy and apoptosis is controversial, since autophagy is sometimes treated as a mechanism that suppresses tumor development or as a tumor growth factor, providing substrates for the degradation of cell components. It has been proposed that, depending on the extension of the damage to the membranes or organelles such as mitochondria and lysosomes, autophagy causes either cell death or regeneration. If damage occurs in the membranes of the two organelles, then this effect may be associated with cell death[49].
Beclin-1, which is encoded by the BECN1 gene, plays an important role in the activation of autophagy and the regulation of multiple cellular signaling pathways of tumors[50,51]. The expression of the BECN1 gene after treatment with 0.3 to 16.6 μmol/L cisplatin did not vary, suggesting that autophagy did not occur. In this sense, it has been shown that downregulating BECN1 resensitized drug-resistant cells to chemotherapy, when autophagy was inhibited and apoptosis was induced[52]. Nevertheless, 33.3 μmol/L cisplatin induced a significant increase in BECN1 expression, meaning that autophagy was more significantly activated after exposure to high concentrations of the compound. Autophagy may be induced by antitumor therapies, and is evaluated as a prosurvival strategy to prevent cell death. Moreover, the induction of autophagic cell death has been proposed as a possible tumor suppression mechanism[53]. Similarly to the present study, it was shown that sepantronium bromide (YM155), a selective survivin target, increases apoptotic and autophagic cell death in human head neck cancer cell lines SCC4, SCC9, SCC15, SCC25, and CAL27[54].
In conclusion, all concentrations of cisplatin used were cytotoxic to human NSCLC NCI-H460 cells in a dose-dependent manner. The toxicity of cisplatin IC50 seems to be associated with MN, suggesting genotoxic damage. Cisplatin may induce apoptosis, based on the inhibition of the antiapoptotic BIRC5 gene. Also, high cisplatin concentration (33.3 µmol/L) induced BECN1 upregulation, which can result in enhanced activity of autophagy and drug resistance to treatment.
Declarations
Authors’ contributionsLiterature review, experiments, preparation of the manuscript: Ballestreri É
Experiments, data analysis, preparation of the manuscript: Simon D, Dihl RR
Experiments: de Souza AP, Grott CS, Nabinger DD
Definition of scientific content, data analyses, manuscript review: Grivicich I
Data source and availabilityCorresponding author may be contacted for any data inquiries.
Financial support and sponsorshipThis work is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (PROSUP-181/201) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
Conflicts of interestThere are no conflicts of interest.
Patient consentNot applicable.
Ethics approvalNot applicable.
Copyright© The Author(s) 2018.
REFERENCES
1. World Health Organization. Cancer. February 2018. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/ [Last accessed on 5 Mar 2018].
2. Addonisio G, Morana G. Lung cancer screening: still not the definitive answer. J Radiol Radiat Ther 2014;2:1-3.
3. Karczmarek-Borowska B, Pelc M, Rabiej E, Grądalska-Lampart M. The quality of life of non-small cell lung cancer patients treated with chemotherapy. Pneumonol Alergol Pol 2014;82:349-57.
4. Zhong C, Liu H, Jiang L, Zhang W, Yao F. Chemotherapy plus best supportive care versus best supportive care in patients with non-small cell lung cancer: a meta-analysis of randomized controlled trials. PLoS One 2013;8:e58466.
5. Raparia K, Villa C, DeCamp MM, Patel JD, Mehta MP. Molecular profiling in non-small cell lung cancer. Arch Pathol Lab Med 2013;137:481-91.
6. Langer CJ, Mok T, Postmus PE. Targeted in the third-/fourth-line treatment of patients with advanced (stage III/IV) non-small-cell lung cancer (NSCLC). Cancer Treat Rev 2013;39:252-60.
7. Cosaert J, Quoix E. Platinum drugs in the treatment of non-small-cell lung cancer. Br J Cancer 2002;87:825-33.
8. Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther 2018;18:63-70.
9. Spinello A, Magistrato A. An omics perspective to the molecular mechanisms of anticancer metallo-drugs in the computational microscope era. Expert Opin Drug Discov 2017;12:813-25.
10. Gebel T, Lantzsch H, Plessow K, Dunkelberg H. Genotoxicity of platinum and palladium compounds in human and bacterial cells. Mut Res 1997;389:183-90.
11. Rjiba-Touati K, Ayed-Boussema I, Skhiri H, Belarbia A, Zellema D, Achour A, Bacha H. Induction of DNA fragmentation, chromosome aberrations and micronuclei by cisplatin in rat bone-marrow cells: protective effect of recombinant human erythropoietin. Mut Res 2012;747:202-6.
12. Bowden NA. Nucleotide excision repair: why is it not used to predict response to platinum-based chemotherapy? Cancer Lett 2014;346:163-71.
13. Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 2007;33:9-23.
14. Yang L, Du C, Wu L, Yu J, An X, Yu W, Cao S, Li H, Ren X. Cytokine-induced killer cells modulates resistance to cisplatin in the A549/DDP cell line. J Cancer 2017;8:3287-95.
15. Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene 2012;31:1869-83.
16. Lin JF, Lin YC, Tsai TF, Chen HE, Chou KY, Hwang TI. Cisplatin induces protective autophagy through activation of BECN1 in human bladder cancer cells. Drug Des Devel Ther 2017;11:1517-33.
17. Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of asoluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 1988;48:4827-33.
18. Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, Minor L. Cell Viability Assays. In: Sittampalam GS, Coussens NP, Brimacombe K, Grossman A, Arkin M, Auld D, Austin C, Baell J, Bejcek B, Chung TDY, Dahlin JL, Devanaryan V, Foley TL, Glicksman M, Hall MD, Hass JV, Inglese J, Iversen PW, Kahl SD, Kales SC, Lal-Nag M, Li Z, McGee J, McManus O, Riss T, Trask OJ Jr, Weidner JR, Xia M, Xu X, editors. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004.
19. Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc 2006;1:2315-9.
21. Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 1990;28:495-503.
22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402-8.
23. Barr MP, Gray SG, Hoffmann AC, Hilger RA, Thomale J, O'Flaherty JD, Fennell DA, Richard D, O'Leary JJ, O'Byrne KJ. Generation and characterisation of cisplatin-resistant non-small cell lung cancer cell lines displaying a stem-like signature. PLoS One 2013;8:e54193.
24. Ferri N, Cazzaniga S, Mazzarella L, Curigliano G, Lucchini G, Zerla D, Gandolfi R, Facchetti G, Pellizzoni M, Rimoldi I. Cytotoxic effect of (1-methyl-1H-imidazol-2-yl)-methanamine and its derivatives in Pt(II) complexes on human carcinoma cell lines: a comparative study with cisplatin. Bioorg Med Chem 2013;21:2379-86.
25. Luo S, Ma K, Zhu H, Wang S, Liu M, Zhang W, Liang S, Xu N. Molecular, biological characterization and drug sensitivity of chidamide-resistant non-small cell lung cancer cells. Oncol Lett 2017;14:6869-75.
26. Lewis CW, Golsteyn RM. Cancer cells that survive checkpoint adaptation contain micronuclei that harbor damaged DNA. Cell Cycle 2016;15:3131-45.
27. Regenthal R, Krueger M, Koeppel C, Preiss R. Drug levels: therapeutic and toxic serum/plasma concentrations of common drugs. J Clin Monit 1999;5:529-44.
28. Tegeder I, Bräutigam L, Seegel M, Al-Dam A, Turowski B, Geisslinger G, Kovács AF. Cisplatin tumor concentrations after intra-arterial cisplatin infusion or embolization in patients with oral cancer. Clin Pharmacol Ther 2003;73:417-26.
29. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 2013;13:1-7.
30. da Silva GN, de Castro Marcondes JP, de Camargo EA, da Silva Passos Júnior GA, Sakamoto-Hojo ET, Salvadori DM. Cell cycle arrest and apoptosis in TP53 subtypes of bladder carcinoma cell lines treated with cisplatin and gemcitabine. Exp Biol Med 2010;235:814-24.
31. Tian L, Zhang J, Ren X, Liu X, Gao W, Zhang C, Sun Y, Liu M. Overexpression of miR-26b decreases the cisplatin-resistance in laryngeal cancer by targeting ATF2. Oncotarget 2017;8:79023-33.
32. Steen NV, Potze L, Giovannetti E, Cavazzoni A, Ruijtenbeek R, Rolfo C, Pauwels P, Peters GJ. Molecular mechanism underlying the pharmacological interactions of the protein kinase C-β inhibitor enzastaurin and erlotinib in non-small cell lung cancer cells. Am J Cancer Res 2017;7:816-30.
33. Miyamoto M, Takano M, Aoyama T, Soyama H, Ishibashi H, Kato K, Iwahashi H, Takasaki K, Kuwahara M, Matuura H, Sakamoto T, Yoshikawa T, Furuya K. Phenoxodiol increases cisplatin sensitivity in ovarian clear cancer cells through XIAP down-regulation and autophagy inhibition. Anticancer Res 2018;38:301-6.
34. Herzog E, Casey A, Lyng FM, Chambers G, Byrne HJ, Davoren M. A new approach to the toxicity testing of carbon-based nanomaterials--the clonogenic assay. Toxicol Lett 2007;174:49-60.
35. Toulany M, Mihatsch J, Holler M, Chaachouay H, Rodemann HP. Cisplatin-mediated radiosensitization of non-small cell lung cancer cells is stimulated by ATM inhibition. Radiother Oncol 2014;111:228-36.
36. Liang H, Wang HB, Liu HZ, Wen XJ, Zhou QL, Yang CX. The effects of combined treatment with sevoflurane and cisplatin on growth and invasion of human adenocarcinoma cell line A549. Biomed Pharmacother 2013;67:503-9.
37. OECD Guidelines for the Testing of Chemicals. In vitro mammalian cell micronucleus test. Adopted: 26 Sep 2014. Available from: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd-tg487-2014-508.pdf [Last accessed on 6 Mar 2018].
38. Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev 1999;99:2467-98.
39. Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003;22:7265-79.
40. Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998;396:580-4.
41. Wang HQ, Jin JJ, Wang J. Arctigenin enhances chemosensitivity to cisplatin in human nonsmall lung cancer H460 cells through downregulation of survivin expression. J Biochem Mol Toxicol 2014;28:39-45.
42. Wong JC, Bathina M, Fiscus RR. Cyclic GMP/protein kinase G type-Iα (PKG-Iα) signaling pathway promotes CREB phosphorylation and maintains higher c-IAP1, livin, survivin, and Mcl-1 expression and the inhibition of PKG-Iα kinase activity synergizes with cisplatin in non-small cell lung cancer cells. J Cell Biochem 2012;113:3587-98.
43. Ueno T, Uehara S, Nakahata K, Okuyama H. Survivin selective inhibitor YM155 promotes cisplatin induced apoptosis in embryonal rhabdomyosarcoma. Int J Oncol 2016;48:1847-54.
44. Alotaibi AAA, Najafzadeh M, Davies JD, Baumgartner A, Anderson D. Inhibition of survivin expression after using oxaliplatin and vinflunine to induce cytogenetic damage in vitro in lymphocytes from colon cancer patients and healthy individuals. Mutagenesis 2017;32:517-24.
45. Wu X, Feng X, Zhao X, Ma F, Liu N, Guo H, Li C, Du H, Zhang B. Role of Beclin-1-mediated autophagy in the survival of pediatric leukemia cells. Cell Physiol Biochem 2016;39:1827-36.
46. Zhou Z, You Z. Mesenchymal stem cells alleviate LPS-induced acute lung injury in mice by miR-142a-5p- controlled pulmonary endothelial cell autophagy. Cell Physiol Biochem 2016;38:258-66.
47. Duffy A, Le J, Sausville E, Emadi A. Autophagy modulation: a target for cancer treatment development. Cancer Chemother Pharmacol 2015;75:439-47.
48. Li C, Liu Y, Liu H, Zhang W, Shen C, Cho K, Chen X, Peng F, Bi Y, Hou X, Yang Z, Zheng Z, Wang K, Wang X, Zhang J, Zhong C, Zou H, Zhang X, Zhao S. Impact of autophagy inhibition at different stages on cytotoxic effect of autophagy inducer in glioblastoma cells. Cell Physiol Biochem 2015;35:1303-16.
49. Martins WK, Costa éT, Cruz MC, Stolf BS, Miotto R, Cordeiro RM, Baptista MS. Parallel damage in mitochondrial and lysosomal compartments promotes efficient cell death with autophagy: the case of the pentacyclic triterpenoids. Sci Rep 2015;5:12425.
50. Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ 2009;16:87-93.
51. Zou Z, Wu L, Ding H, Wang Y, Zhang Y, Chen X, Chen X, Zhang CY, Zhang Q, Zen K. MicroRNA-30a sensitizes tumor cells to cis-platinum via suppressing beclin 1-mediated autophagy. J Biol Chem 2012;287:4148-56.
52. Yang X, Bai F, Xu Y, Chen Y, Chen L. Intensified Beclin-1 mediated by low expression of miR-30a-5p promotes chemoresistance in human small cell lung cancer. Cell Physiol Biochem 2017;43:1126-39.
53. Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006;10:51-64.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Ballestreri, Simon D, Souza AP, Grott CS, Nabinger DD, Dihl RR, Grivicich I. Resistance mechanism to cisplatin in NCI-H460 non-small cell lung cancer cell line: investigating apoptosis, autophagy, and cytogenetic damage. Cancer Drug Resist 2018;1:72-81. http://dx.doi.org/10.20517/cdr.2017.02
AMA Style
Ballestreri, Simon D, Souza AP, Grott CS, Nabinger DD, Dihl RR, Grivicich I. Resistance mechanism to cisplatin in NCI-H460 non-small cell lung cancer cell line: investigating apoptosis, autophagy, and cytogenetic damage. Cancer Drug Resistance. 2018; 1(1): 72-81. http://dx.doi.org/10.20517/cdr.2017.02
Chicago/Turabian Style
Ballestreri, Érica, Daniel Simon, Ana Paula de Souza, Camila Schultz Grott, Débora Dreher Nabinger, Rafael Rodrigues Dihl, Ivana Grivicich. 2018. "Resistance mechanism to cisplatin in NCI-H460 non-small cell lung cancer cell line: investigating apoptosis, autophagy, and cytogenetic damage" Cancer Drug Resistance. 1, no.1: 72-81. http://dx.doi.org/10.20517/cdr.2017.02
ACS Style
Ballestreri, Simon D.; Souza AP.; Grott CS.; Nabinger DD.; Dihl RR.; Grivicich I. Resistance mechanism to cisplatin in NCI-H460 non-small cell lung cancer cell line: investigating apoptosis, autophagy, and cytogenetic damage. Cancer Drug Resist. 2018, 1, 72-81. http://dx.doi.org/10.20517/cdr.2017.02
About This Article
Copyright
Data & Comments
Data
 Cite This Article 4 clicks
Cite This Article 4 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.